Evaluation of porcine GM-CSF during PRRSV infection in vitro and in vivo indicating a protective role of GM-CSF related with M1 biased activation in alveolar macrophage during PRRSV infection
- PMID: 36341451
- PMCID: PMC9627285
- DOI: 10.3389/fimmu.2022.967338
Evaluation of porcine GM-CSF during PRRSV infection in vitro and in vivo indicating a protective role of GM-CSF related with M1 biased activation in alveolar macrophage during PRRSV infection
Abstract
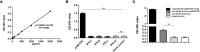
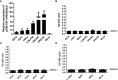
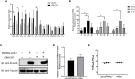
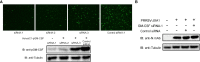
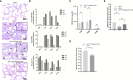
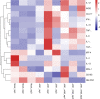
Granulocyte-macrophage colony stimulating factor (GM-CSF), participates in diverse biological processes associated with innate and adaptive immunity, has unknown effects during PRRSV infection. Here, a double-antibody sandwich ELISA for pGM-CSF was developed in-house for evaluation of pGM-CSF level during PRRSV infection both in vitro and in vivo. In in vitro assay, it was notable that PRRSV-infected porcine alveolar macrophages (PAMs) yielded inconsistent pGM-CSF protein- and mRNA-level, suggesting a post-transcriptional inhibition of pGM-CSF mRNA was employed by PRRSV. Meanwhile, concurrent analysis of pGM-CSF levels in serum samples from PRRSV-infected piglets suggested that effect of PRRSV infection demonstrated minimum effect on pGM-CSF levels regardless of PRRSV virulence phenotypes. Moreover, in vitro treatment of PAMs with pGM-CSF prior PRRSV inoculation did not inhibit PRRSV replication in PAMs although genes downstream of pGM-CSF in PAMs could be upregulated by pGM-CSF treatment. Meanwhile, knockdown of pGM-CSF using siRNA did not enhance PRRSV replication as well. Intriguingly, therapeutic antibody treatment of HP-PRRSV-infected piglets led to significantly increased serum pGM-CSF levels, thus aligning with low pneumonia incidence and low intracellular PRRSV-RNA levels in PAMs of therapeutic antibody treated piglets. Furthermore, transcriptome analysis of PAMs from infected piglets revealed increased serum pGM-CSF levels correlated with activation of downstream signal of pGM-CSF in PAMs as evidenced by a M1-like phenotypes of gene expression pattern, implying a potential host-protective role played by pGM-CSF for PRRSV infection in vivo. In conclusion, our results demonstrated developments of a highly sensitive and specific ELISA for pGM-CSF and revealed a potential protective role conferred by pGM-CSF during PRRSV infection.
Keywords: ELISA; GM-CSF; PRRSV; immune response; macrophage activation.
Copyright © 2022 Ji, Qu, Liu, Bai, Wang, Chen, Zheng, Zhang, Yang and Wu.
Conflict of interest statement
Author RC was employed by the company Shaanxi Innolever Biotechnology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Porcine Reproductive and Respiratory Syndrome Virus Infection Upregulates Negative Immune Regulators and T-Cell Exhaustion Markers.J Virol. 2021 Oct 13;95(21):e0105221. doi: 10.1128/JVI.01052-21. Epub 2021 Aug 11. J Virol. 2021. PMID: 34379512 Free PMC article.
-
Molecular characterization of transcriptome-wide interactions between highly pathogenic porcine reproductive and respiratory syndrome virus and porcine alveolar macrophages in vivo.Int J Biol Sci. 2011;7(7):947-59. doi: 10.7150/ijbs.7.947. Epub 2011 Aug 7. Int J Biol Sci. 2011. PMID: 21850204 Free PMC article.
-
Transcriptional Immune Signatures of Alveolar Macrophages and the Impact of the NLRP3 Inflammasome on Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Replication.Viruses. 2020 Nov 12;12(11):1299. doi: 10.3390/v12111299. Viruses. 2020. PMID: 33198300 Free PMC article.
-
Transcriptome Analysis Reveals Dynamic Gene Expression Profiles in Porcine Alveolar Macrophages in Response to the Chinese Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus.Biomed Res Int. 2018 Apr 29;2018:1538127. doi: 10.1155/2018/1538127. eCollection 2018. Biomed Res Int. 2018. PMID: 29854728 Free PMC article.
-
Immune responses of pigs immunized with a recombinant porcine reproductive and respiratory syndrome virus expressing porcine GM-CSF.Vet Immunol Immunopathol. 2015 Nov 15;168(1-2):40-8. doi: 10.1016/j.vetimm.2015.08.003. Epub 2015 Aug 14. Vet Immunol Immunopathol. 2015. PMID: 26300317
Cited by
-
Porcine Reproductive and Respiratory Syndrome Virus Modulates the Switch of Macrophage Polarization from M1 to M2 by Upregulating MoDC-Released sCD83.Viruses. 2023 Mar 17;15(3):773. doi: 10.3390/v15030773. Viruses. 2023. PMID: 36992481 Free PMC article.
-
Paternal genetic effects of cadmium exposure during pregnancy on hormone synthesis disorders in ovarian granulosa cells of offspring.J Ovarian Res. 2023 May 16;16(1):98. doi: 10.1186/s13048-023-01175-5. J Ovarian Res. 2023. PMID: 37194017 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

