Saliva antibody-fingerprint of reactivated latent viruses after mild/asymptomatic COVID-19 is unique in patients with myalgic-encephalomyelitis/chronic fatigue syndrome
- PMID: 36341457
- PMCID: PMC9630598
- DOI: 10.3389/fimmu.2022.949787
Saliva antibody-fingerprint of reactivated latent viruses after mild/asymptomatic COVID-19 is unique in patients with myalgic-encephalomyelitis/chronic fatigue syndrome
Abstract
Background: Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a chronic disease considered to be triggered by viral infections in a majority of cases. Symptoms overlap largely with those of post-acute sequelae of COVID-19/long-COVID implying common pathogenetic mechanisms. SARS-CoV-2 infection is risk factor for sustained latent virus reactivation that may account for the symptoms of post-viral fatigue syndromes. The aim of this study was first to investigate whether patients with ME/CFS and healthy donors (HDs) differed in their antibody response to mild/asymptomatic SARS-CoV-2 infection. Secondly, to analyze whether COVID-19 imposes latent virus reactivation in the cohorts.
Methods: Anti-SARS-CoV-2 antibodies were analyzed in plasma and saliva from non-vaccinated ME/CFS (n=95) and HDs (n=110) using soluble multiplex immunoassay. Reactivation of human herpesviruses 1-6 (HSV1, HSV2, VZV, EBV, CMV, HHV6), and human endogenous retrovirus K (HERV-K) was detected by anti-viral antibody fingerprints in saliva.
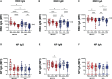
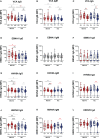
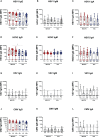
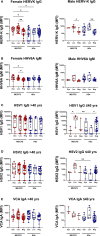
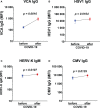
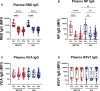
Results: At 3-6 months after mild/asymptomatic SARS-CoV-2 infection, virus-specific antibodies in saliva were substantially induced signifying a strong reactivation of latent viruses (EBV, HHV6 and HERV-K) in both cohorts. In patients with ME/CFS, antibody responses were significantly stronger, in particular EBV-encoded nuclear antigen-1 (EBNA1) IgG were elevated in patients with ME/CFS, but not in HDs. EBV-VCA IgG was also elevated at baseline prior to SARS-infection in patients compared to HDs.
Conclusion: Our results denote an altered and chronically aroused anti-viral profile against latent viruses in ME/CFS. SARS-CoV-2 infection even in its mild/asymptomatic form is a potent trigger for reactivation of latent herpesviruses (EBV, HHV6) and endogenous retroviruses (HERV-K), as detected by antibody fingerprints locally in the oral mucosa (saliva samples). This has not been shown before because the antibody elevation is not detected systemically in the circulation/plasma.
Keywords: COVID-19; EBV; HERV; HHV6A; ME/CFS; herpesvirus reactivation; latent virus; myalgic encephalomyelitis/chronic fatigue syndrome.
Copyright © 2022 Apostolou, Rizwan, Moustardas, Sjögren, Bertilson, Bragée, Polo and Rosén.
Conflict of interest statement
PS, BCB, BB, and OP declare disclosure of interest as having income from Bragée Clinics, and BB being a partial owner. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Post-COVID sequelae effect in chronic fatigue syndrome: SARS-CoV-2 triggers latent adenovirus in the oral mucosa.Front Med (Lausanne). 2023 Jun 29;10:1208181. doi: 10.3389/fmed.2023.1208181. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37457558 Free PMC article.
-
Prevalence of EBV, HHV6, HCMV, HAdV, SARS-CoV-2, and Autoantibodies to Type I Interferon in Sputum from Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients.Viruses. 2025 Mar 14;17(3):422. doi: 10.3390/v17030422. Viruses. 2025. PMID: 40143349 Free PMC article.
-
Persistent SARS-CoV-2 Infection, EBV, HHV-6 and Other Factors May Contribute to Inflammation and Autoimmunity in Long COVID.Viruses. 2023 Jan 31;15(2):400. doi: 10.3390/v15020400. Viruses. 2023. PMID: 36851614 Free PMC article. Review.
-
Cytomegalovirus, Epstein-Barr virus, and human herpesvirus-6 infections in patients with myalgic еncephalomyelitis/chronic fatigue syndrome.J Med Virol. 2020 Dec;92(12):3682-3688. doi: 10.1002/jmv.25744. Epub 2020 Mar 11. J Med Virol. 2020. PMID: 32129496 Free PMC article.
-
Cardiovascular and haematological pathology in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): A role for viruses.Blood Rev. 2023 Jul;60:101075. doi: 10.1016/j.blre.2023.101075. Epub 2023 Mar 20. Blood Rev. 2023. PMID: 36963989 Free PMC article. Review.
Cited by
-
Inflammation and Epstein-Barr Virus at the Crossroads of Multiple Sclerosis and Post-Acute Sequelae of COVID-19 Infection.Viruses. 2023 Apr 12;15(4):949. doi: 10.3390/v15040949. Viruses. 2023. PMID: 37112929 Free PMC article. Review.
-
Core features and inherent diversity of post-acute infection syndromes.Front Immunol. 2025 Jun 3;16:1509131. doi: 10.3389/fimmu.2025.1509131. eCollection 2025. Front Immunol. 2025. PMID: 40529374 Free PMC article. Review.
-
Post-acute sequelae of SARS-CoV-2 infection (Long COVID) in older adults.Geroscience. 2024 Dec;46(6):6563-6581. doi: 10.1007/s11357-024-01227-8. Epub 2024 Jun 14. Geroscience. 2024. PMID: 38874693 Free PMC article. Review.
-
Over-Representation of Torque Teno Mini Virus 9 in a Subgroup of Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Pilot Study.Pathogens. 2024 Sep 1;13(9):751. doi: 10.3390/pathogens13090751. Pathogens. 2024. PMID: 39338942 Free PMC article.
-
The immunology of long COVID.Nat Rev Immunol. 2023 Oct;23(10):618-634. doi: 10.1038/s41577-023-00904-7. Epub 2023 Jul 11. Nat Rev Immunol. 2023. PMID: 37433988 Review.
References
-
- Hickie I, Davenport T, Wakefield D, Vollmer-Conna U, Cameron B, Vernon SD, et al. . Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. Bmj (2006) 333:575. doi: 10.1136/bmj.38933.585764.AE - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

