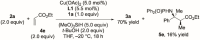Diastereo- and Enantioselective Reductive Mannich-type Reaction of α,β-Unsaturated Carboxylic Acids to Ketimines: A Direct Entry to Unprotected β2,3,3 -Amino Acids
- PMID: 36341524
- PMCID: PMC10107894
- DOI: 10.1002/chem.202202575
Diastereo- and Enantioselective Reductive Mannich-type Reaction of α,β-Unsaturated Carboxylic Acids to Ketimines: A Direct Entry to Unprotected β2,3,3 -Amino Acids
Abstract
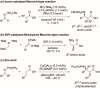
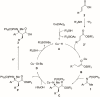
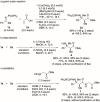
Stereoselective construction of unprotected β-amino acids is a significant challenge owing to the lack of methods for the catalytic generation of highly enantioenriched carboxylic acid enolates. In this study, a novel copper-catalyzed diastereo- and enantioselective reductive Mannich-type reaction of α,β-unsaturated carboxylic acids was developed, which provides a direct and scalable synthetic method for enantioenriched β2,3,3 -amino acids with vicinal stereogenic centers. The protocol features in situ generation of transiently protected carboxylic acids by a hydrosilane and their diastereo- and enantioselective reductive coupling with ketimines. The synthetic utility of this process was demonstrated by a gram-scale reaction and the transformation of β-amino acids.
Keywords: amino acids; asymmetric catalysis; carboxylic acids; copper; reductive Mannich-type reaction.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Copper-Catalyzed Enantioselective Reductive Aldol Reaction of α,β-Unsaturated Carboxylic Acids to Alkyl Aryl Ketones: Silanes as Activator and Transient Protecting Group.Chemistry. 2022 Feb 16;28(9):e202104273. doi: 10.1002/chem.202104273. Epub 2022 Jan 21. Chemistry. 2022. PMID: 34967961
-
Direct Access to N-Unprotected α- and/or β-Tetrasubstituted Amino Acid Esters via Direct Catalytic Mannich-Type Reactions Using N-Unprotected Trifluoromethyl Ketimines.Chemistry. 2017 Dec 1;23(67):17022-17028. doi: 10.1002/chem.201703516. Epub 2017 Sep 26. Chemistry. 2017. PMID: 28950035
-
Catalytic Asymmetric Mannich Reaction of α-Fluoronitriles with Ketimines: Enantioselective and Diastereodivergent Construction of Vicinal Tetrasubstituted Stereocenters.ACS Catal. 2019 Mar 1;9(3):2169-2176. doi: 10.1021/acscatal.8b05164. Epub 2019 Feb 6. ACS Catal. 2019. PMID: 30956891 Free PMC article.
-
Enantioselective total syntheses of several bioactive natural products based on the development of practical asymmetric catalysis.Chem Pharm Bull (Tokyo). 2004 Sep;52(9):1031-52. doi: 10.1248/cpb.52.1031. Chem Pharm Bull (Tokyo). 2004. PMID: 15340187 Review.
-
Catalytic asymmetric synthesis of α-stereogenic carboxylic acids: recent advances.Org Biomol Chem. 2021 Dec 22;20(1):37-54. doi: 10.1039/d1ob02038b. Org Biomol Chem. 2021. PMID: 34854454 Review.
Cited by
-
Regio- and Enantioconvergent Hydroallylation of Acrylates Enabled by γ-Silyl-Substituted Allyl Acetates.Angew Chem Int Ed Engl. 2025 May;64(19):e202425256. doi: 10.1002/anie.202425256. Epub 2025 Apr 21. Angew Chem Int Ed Engl. 2025. PMID: 40044597 Free PMC article.
References
-
- For selected reviews, see:
-
- Cheng R. P., Gellman S. H., DeGrado W. F., Chem. Rev. 2001, 101, 3219; - PubMed
-
- Lelais G., Seebach D., Biopolymers 2004, 76, 206; - PubMed
-
- Seebach D., Gardiner J., Acc. Chem. Res. 2008, 41, 1366; - PubMed
-
- Kudo F., Miyanaga A., Eguchi T., Nat. Prod. Rep. 2014, 31, 1056; - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources