Super-resolution imaging uncovers the nanoscopic segregation of polarity proteins in epithelia
- PMID: 36341714
- PMCID: PMC9674336
- DOI: 10.7554/eLife.62087
Super-resolution imaging uncovers the nanoscopic segregation of polarity proteins in epithelia
Abstract
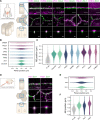
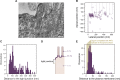
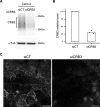
Epithelial tissues acquire their integrity and function through the apico-basal polarization of their constituent cells. Proteins of the PAR and Crumbs complexes are pivotal to epithelial polarization, but the mechanistic understanding of polarization is challenging to reach, largely because numerous potential interactions between these proteins and others have been found, without a clear hierarchy in importance. We identify the regionalized and segregated organization of members of the PAR and Crumbs complexes at epithelial apical junctions by imaging endogenous proteins using stimulated-emission-depletion microscopy on Caco-2 cells, and human and murine intestinal samples. Proteins organize in submicrometric clusters, with PAR3 overlapping with the tight junction (TJ) while PALS1-PATJ and aPKC-PAR6β form segregated clusters that are apical of the TJ and present in an alternated pattern related to actin organization. CRB3A is also apical of the TJ and partially overlaps with other polarity proteins. Of the numerous potential interactions identified between polarity proteins, only PALS1-PATJ and aPKC-PAR6β are spatially relevant in the junctional area of mature epithelial cells, simplifying our view of how polarity proteins could cooperate to drive and maintain cell polarity.
Keywords: Caco-2 cells; STED; cell biology; cell polarity; human; human intestine; mouse; polarity proteins.
Plain language summary
Many of our organs, including the lungs and the intestine, are lined with a single layer of cells that separate the inside of the organ from the surrounding environment inside the body. These so-called epithelial cells form a tightly packed barrier and have a very characteristic organization. The apical surface faces the outside world, while the basal surface faces the inner tissues. These different interfaces are reflected in the organization of the cells themselves. The shape, composition, and role of the apical cell surface are distinct from those of the basal surface, and they also contain different proteins. In some epithelial cells, the apical surface specializes and forms protruding structures called microvilli. Thus, epithelial cells are said to be polarized along this apical–basal axis. Over the last 30 years, many labs have identified and studied which proteins help epithelial cells become and stay polarized. Previous biochemical experiments showed that these so-called polarity proteins interact with each other in many different ways. But it remains unclear whether some of these interactions are more important than others, and where exactly in the apical or basal membranes these interactions take place. Mangeol et al. used super-resolution microscopy to observe the polarity of proteins at the apical membranes of both human and mouse cells from the small intestine to answer these questions. They focused on areas called tight junctions, where the intestinal cells connect with each other to form the barrier between the outside and the inside. First, all the polarity proteins clustered together in various formations, they were not distributed uniformly. For example, one protein called PAR3 was at the level of the tight junctions, whereas other proteins were closer to the apical surface and the outside world. Only two pairs of proteins – PAR6 and aPKC, and PALS1 and PATJ – formed stable clusters with each other. This finding was unexpected because previous biochemical experiments had predicted multiple interactions. Third, the PALS1/PATJ complexes stayed at the bottom of the microvilli protrusions, whereas PAR6/aPKC were inside the protrusions. Taken together, these experiments reveal a detailed snapshot of how the polarity proteins themselves are organized at the apical surface of epithelial cells. Future work will be able to address how these protein complexes behave over time.
© 2022, Mangeol et al.
Conflict of interest statement
PM, DM, FR, JC, PL, AL No competing interests declared
Figures









Similar articles
-
Direct interaction of two polarity complexes implicated in epithelial tight junction assembly.Nat Cell Biol. 2003 Feb;5(2):137-42. doi: 10.1038/ncb923. Nat Cell Biol. 2003. PMID: 12545177
-
The Mammalian Crumbs Complex Defines a Distinct Polarity Domain Apical of Epithelial Tight Junctions.Curr Biol. 2020 Jul 20;30(14):2791-2804.e6. doi: 10.1016/j.cub.2020.05.032. Epub 2020 Jun 11. Curr Biol. 2020. PMID: 32531288
-
Role of the Crumbs proteins in ciliogenesis, cell migration and actin organization.Semin Cell Dev Biol. 2018 Sep;81:13-20. doi: 10.1016/j.semcdb.2017.10.018. Epub 2017 Oct 24. Semin Cell Dev Biol. 2018. PMID: 29056580 Review.
-
PATJ regulates tight junction formation and polarity in mammalian epithelial cells.J Cell Biol. 2005 Feb 28;168(5):705-11. doi: 10.1083/jcb.200408064. J Cell Biol. 2005. PMID: 15738264 Free PMC article.
-
Polarity complex proteins.Biochim Biophys Acta. 2008 Mar;1778(3):614-30. doi: 10.1016/j.bbamem.2007.08.029. Epub 2007 Sep 12. Biochim Biophys Acta. 2008. PMID: 18005931 Review.
Cited by
-
A neuroligin-2-YAP axis regulates progression of pancreatic intraepithelial neoplasia.EMBO Rep. 2024 Apr;25(4):1886-1908. doi: 10.1038/s44319-024-00104-x. Epub 2024 Feb 27. EMBO Rep. 2024. PMID: 38413734 Free PMC article.
-
The zonula adherens matura redefines the apical junction of intestinal epithelia.Proc Natl Acad Sci U S A. 2024 Feb 27;121(9):e2316722121. doi: 10.1073/pnas.2316722121. Epub 2024 Feb 20. Proc Natl Acad Sci U S A. 2024. PMID: 38377188 Free PMC article.
-
Membrane prewetting by condensates promotes tight-junction belt formation.Nature. 2024 Aug;632(8025):647-655. doi: 10.1038/s41586-024-07726-0. Epub 2024 Aug 7. Nature. 2024. PMID: 39112699 Free PMC article.
-
The Nanoscopic Organization of Synapse Structures: A Common Basis for Cell Communication.Membranes (Basel). 2021 Mar 30;11(4):248. doi: 10.3390/membranes11040248. Membranes (Basel). 2021. PMID: 33808285 Free PMC article. Review.
-
Loss of intermicrovillar adhesion factor CDHR2 impairs basolateral junctional complexes in transporting epithelia.Mol Biol Cell. 2024 Nov 1;35(11):br21. doi: 10.1091/mbc.E24-03-0113. Epub 2024 Sep 18. Mol Biol Cell. 2024. PMID: 39292922 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

