Social isolation exacerbates acute ozone inhalation induced pulmonary and systemic health outcomes
- PMID: 36341779
- PMCID: PMC9722630
- DOI: 10.1016/j.taap.2022.116295
Social isolation exacerbates acute ozone inhalation induced pulmonary and systemic health outcomes
Abstract
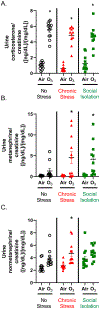
Psychosocially-stressed individuals might have exacerbated responses to air pollution exposure. Acute ozone exposure activates the neuroendocrine stress response leading to systemic metabolic and lung inflammatory changes. We hypothesized chronic mild stress (CS) and/or social isolation (SI) would cause neuroendocrine, inflammatory, and metabolic phenotypes that would be exacerbated by an acute ozone exposure. Male 5-week-old Wistar-Kyoto rats were randomly assigned into 3 groups: no stress (NS) (pair-housed, regular-handling); SI (single-housed, minimal-handling); CS (single-housed, subjected to mild unpredicted-randomized stressors [restraint-1 h, tilted cage-1 h, shaking-1 h, intermittent noise-6 h, and predator odor-1 h], 1-stressor/day*5-days/week*8-weeks. All animals then 13-week-old were subsequently exposed to filtered-air or ozone (0.8-ppm) for 4 h and immediately necropsied. CS, but not SI animals had increased adrenal weights. However, relative to NS, both CS and SI had lower circulating luteinizing hormone, prolactin, and follicle-stimulating hormone regardless of exposure (SI > CS), and only CS demonstrated lower thyroid-stimulating hormone levels. SI caused more severe systemic inflammation than CS, as evidenced by higher circulating cytokines and cholesterol. Ozone exposure increased urine corticosterone and catecholamine metabolites with no significant stressor effect. Ozone-induced lung injury, and increases in lavage-fluid neutrophils and IL-6, were exacerbated by SI. Ozone severely lowered circulating thyroid-stimulating hormone, prolactin, and luteinizing hormone in all groups and exacerbated systemic inflammation in SI. Ozone-induced increases in serum glucose, leptin, and triglycerides were consistent across stressors; however, increases in cholesterol were exacerbated by SI. Collectively, psychosocial stressors, especially SI, affected the neuroendocrine system and induced adverse metabolic and inflammatory effects that were exacerbated by ozone exposure.
Keywords: Catecholamines; Glucocorticoids; Mild chronic stress model; Neuroendocrine; Ozone; Pituitary hormones; Social isolation; Stress response; Systemic inflammation.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







Similar articles
-
Influence of Mild Chronic Stress and Social Isolation on Acute Ozone-Induced Alterations in Stress Biomarkers and Brain-Region-Specific Gene Expression in Male Wistar-Kyoto Rats.Antioxidants (Basel). 2023 Nov 3;12(11):1964. doi: 10.3390/antiox12111964. Antioxidants (Basel). 2023. PMID: 38001817 Free PMC article.
-
The contribution of the neuroendocrine system to adaption after repeated daily ozone exposure in rats.Toxicol Appl Pharmacol. 2022 Jul 15;447:116085. doi: 10.1016/j.taap.2022.116085. Epub 2022 May 23. Toxicol Appl Pharmacol. 2022. PMID: 35618032 Free PMC article.
-
Beta-2 Adrenergic and Glucocorticoid Receptor Agonists Modulate Ozone-Induced Pulmonary Protein Leakage and Inflammation in Healthy and Adrenalectomized Rats.Toxicol Sci. 2018 Dec 1;166(2):288-305. doi: 10.1093/toxsci/kfy198. Toxicol Sci. 2018. PMID: 30379318 Free PMC article.
-
Neuroendocrine Regulation of Air Pollution Health Effects: Emerging Insights.Toxicol Sci. 2018 Jul 1;164(1):9-20. doi: 10.1093/toxsci/kfy129. Toxicol Sci. 2018. PMID: 29846720 Free PMC article. Review.
-
Inhalation of environmental stressors & chronic inflammation: autoimmunity and neurodegeneration.Mutat Res. 2009 Mar 31;674(1-2):62-72. doi: 10.1016/j.mrgentox.2008.09.016. Epub 2008 Oct 11. Mutat Res. 2009. PMID: 18977456 Free PMC article. Review.
Cited by
-
Influence of Mild Chronic Stress and Social Isolation on Acute Ozone-Induced Alterations in Stress Biomarkers and Brain-Region-Specific Gene Expression in Male Wistar-Kyoto Rats.Antioxidants (Basel). 2023 Nov 3;12(11):1964. doi: 10.3390/antiox12111964. Antioxidants (Basel). 2023. PMID: 38001817 Free PMC article.
-
Multi-tissue transcriptomic and serum metabolomic assessment reveals systemic implications of acute ozone-induced stress response in male Wistar Kyoto rats.Metabolomics. 2023 Sep 10;19(9):81. doi: 10.1007/s11306-023-02043-5. Metabolomics. 2023. PMID: 37690105 Free PMC article.
-
Effects of Ambient O3 on Respiratory Mortality, Especially the Combined Effects of PM2.5 and O3.Toxics. 2023 Oct 30;11(11):892. doi: 10.3390/toxics11110892. Toxics. 2023. PMID: 37999544 Free PMC article.
-
Mechanistic insights regarding neuropsychiatric and neuropathologic impacts of air pollution.Crit Rev Toxicol. 2024 Nov;54(10):953-980. doi: 10.1080/10408444.2024.2420972. Epub 2024 Dec 10. Crit Rev Toxicol. 2024. PMID: 39655487 Review.
References
-
- Arcego DM, Krolow R, Lampert C, Noschang C, Ferreira AG, Scherer E, Wyse AT, Dalmaz C. Isolation during the prepubertal period associated with chronic access to palatable diets: effects on plasma lipid profile and liver oxidative stress. Physiol Behav. 2014. Jan 30;124:23–32. doi: 10.1016/j.physbeh.2013.10.029. Epub 2013 Oct 30. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

