Prolonged, physiologically relevant nicotine concentrations in the airways of smokers
- PMID: 36342131
- PMCID: PMC9829458
- DOI: 10.1152/ajplung.00038.2022
Prolonged, physiologically relevant nicotine concentrations in the airways of smokers
Abstract
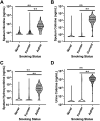
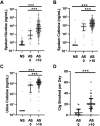
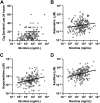
Nicotine from cigarette smoke is a biologically active molecule that has pleiotropic effects in the airway, which could play a role in smoking-induced lung disease. However, whether nicotine and its metabolites reach sustained, physiologically relevant concentrations on airway surfaces of smokers is not well defined. To address these issues, concentrations of nicotine, cotinine, and hydroxycotinine were measured by mass spectrometry (MS) in supernatants of induced sputum obtained from participants in the subpopulations and intermediate outcome measures in COPD study (SPIROMICS), an ongoing observational study that included never smokers, former smokers, and current smokers with and without chronic obstructive pulmonary disease (COPD). A total of 980 sputum supernatants were analyzed from 77 healthy never smokers, 494 former smokers (233 with COPD), and 396 active smokers (151 with COPD). Sputum nicotine, cotinine, and hydroxycotinine concentrations corresponded to self-reported smoking status and were strongly correlated to urine measures. A cutoff of ∼8-10 ng/mL of sputum cotinine distinguished never smokers from active smokers. Accounting for sample dilution during processing, active smokers had airway nicotine concentrations in the 70-850 ng/mL (∼0.5-5 µM) range, and concentrations remained elevated even in current smokers who had not smoked within 24 h. This study demonstrates that airway nicotine and its metabolites are readily measured in sputum supernatants and can serve as biological markers of smoke exposure. In current smokers, nicotine is present at physiologically relevant concentrations for prolonged periods, supporting a contribution to cigarette-induced airway disease.
Keywords: COPD; cotinine; sputum.
Conflict of interest statement
C.B.C. reports personal fees from PulmonX, other from GlaxoSmithKline, personal fees from NUVAIRA, and personal fees from MGC Diagnostics. I.B. has received grants from Amgen, GE Healthcare, Aerogen, Theravance, and Viatris and has received consulting fees from Astra Zeneca, GSK, Theravance, Viatris, Grifols, Inhibrx. L.M.R. is a consultant for the TOPMed Administrative Coordinating Center (through WeStat). R.P.B. reports grants and personal fees from Boehringer Ingelheim, personal fees from Mylan Pharmaceuticals, personal fees from Theravance, grants, personal fees, and nonfinancial support from GSK. A.P.C. reports personal fees from GSK, nonfinancial support from VIDA. J.L.C. reports personal fees from AstraZeneca and CSL Behring. V.E.O. reports personal fees for serving on Independent Data Monitoring Committees for Regeneron and Sanofi. J.M.W. reports grants from Bayer, grants and other from GSK, other from Boehringer Ingelheim, grants and other from Mereo BioPharma, other from PRA. R.C.B. reports receiving fees from Parion Sciences. All disclosures are outside of the submitted work. See
Figures

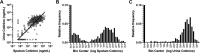
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

