Efficacy and Immune Correlates of OMP-1B and VirB2-4 Vaccines for Protection of Dogs from Tick Transmission of Ehrlichia chaffeensis
- PMID: 36342170
- PMCID: PMC9765013
- DOI: 10.1128/mbio.02140-22
Efficacy and Immune Correlates of OMP-1B and VirB2-4 Vaccines for Protection of Dogs from Tick Transmission of Ehrlichia chaffeensis
Abstract
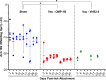
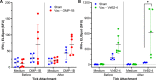
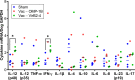
Ehrlichia chaffeensis, an obligatory intracellular bacterium, causes human monocytic ehrlichiosis, an emerging disease transmitted by the Lone Star tick, Amblyomma americanum. Here, we investigated the vaccine potential of OMP-1B and VirB2-4. Among the highly expressed and immunodominant E. chaffeensis porin P28s/OMP-1s, OMP-1B is predominantly expressed by E. chaffeensis in A. americanum ticks, whereas VirB2-4 is a pilus protein of the type IV secretion system essential for E. chaffeensis infection of host cells. Immunization with recombinant OMP-1B (rOMP-1B) or recombinant VirB2-4 (rVirB2-4) protected mice from E. chaffeensis infection as effectively as Entry-triggering protein of Ehrlichia immunization. Dogs vaccinated with a nanoparticle vaccine composed of rOMP-1B or rVirB2-4 and an immunostimulating complex developed high antibody titers against the respective antigen. Upon challenge with E. chaffeensis-infected A. americanum ticks, E. chaffeensis was undetectable in the blood of rOMP-1B or rVirB2-4 immunized dogs on day 3 or 6 post-tick attachment and for the duration of the experiment, whereas dogs sham-vaccinated with the complex alone were persistently infected for the duration of the experiment. E. chaffeensis exponentially replicates in blood-feeding ticks to facilitate transmission. Previously infected ticks removed from OMP-1B-immunized dogs showed significantly lower bacterial load relative to ticks removed from sham-immunized dogs, suggesting in-tick neutralization. Peripheral blood leukocytes from rVirB2-4-vaccinated dogs secreted significantly elevated amounts of interferon-γ soon after tick attachment by ELISpot assay and reverse transcription-quantitative PCR, suggesting interferon-γ-mediated Ehrlichia inhibition. Thus, Ehrlichia surface-exposed proteins OMP-1B and VirB2-4 represent new potential vaccine candidates for blocking tick-borne ehrlichial transmission. IMPORTANCE Ehrlichia are tick-borne pathogens that cause a potentially fatal illness-ehrlichiosis-in animals and humans worldwide. Currently, no vaccine is available for ehrlichiosis, and treatment options are limited. Ticks are biological vectors of Ehrlichia, i.e., Ehrlichia exponentially replicates in blood-sucking ticks before infecting animals. Ticks also inoculate immunomodulatory substances into animals. Thus, it is important to study effects of candidate vaccines on Ehrlichia infection in both animals and ticks and the immune responses of animals shortly after infected tick challenge. Here, we investigated the efficacy of vaccination with functionality-defined two surface-exposed outer membrane proteins of Ehrlichia chaffeensis, OMP-1B and VirB2-4, in a mouse infection model and then in a dog-tick transmission model. Our results begin to fill gaps in our understanding of Ehrlichia-derived protective antigens against tick-transmission and immune correlates and mechanisms that could help future development of vaccines for immunization of humans and animals to counter tick-transmitted ehrlichiosis.
Keywords: Ehrlichia; IFN-γ; ISCOM; OMP-1B; VirB2-4; dog; in-tick neutralization; tick transmission; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
