Hungatella hathewayi, an Efficient Glycosaminoglycan-Degrading Firmicutes from Human Gut and Its Chondroitin ABC Exolyase with High Activity and Broad Substrate Specificity
- PMID: 36342199
- PMCID: PMC9680638
- DOI: 10.1128/aem.01546-22
Hungatella hathewayi, an Efficient Glycosaminoglycan-Degrading Firmicutes from Human Gut and Its Chondroitin ABC Exolyase with High Activity and Broad Substrate Specificity
Abstract
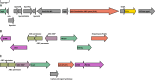
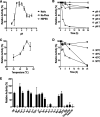
The degradation of glycosaminoglycans (GAGs) by intestinal bacteria is critical for their colonization in the human gut and the health of the host. Both colonic Bacteroides and Firmicutes have been reported to degrade GAGs; however, the enzymatic details of the latter remain largely unknown. Our bioinformatic analyses of fecal Firmicutes revealed that their genomes, especially Hungatella hathewayi strains, are an abundant source of putative GAG-specific catabolic enzymes. Subsequently, we isolated a Firmicutes strain, H. hathewayi N2-326, that can catabolize various GAGs. While H. hathewayi N2-326 was as efficient in utilizing chondroitin sulfate A (CSA) and dermatan sulfate as Bacteroides thetaiotaomicron, a well-characterized GAG degrader, it outperformed B. thetaiotaomicron in assimilating hyaluronic acid. Unlike B. thetaiotaomicron, H. hathewayi N2-326 could not utilize heparin. The chondroitin lyase activity of H. hathewayi N2-326 was found to be present predominantly in the culture supernatant. Genome sequence analysis revealed three putative GAG lyases, but only the HH-chondroitin ABC lyase was upregulated in the presence of CSA. In addition, five CAZyme gene clusters containing GAG metabolism genes were significantly upregulated when grown on CSA. Further characterization of the recombinant HH-chondroitin ABC lyase revealed that it cleaves GAGs predominantly in an exo-mode to produce unsaturated disaccharides as the primary hydrolytic product while exhibiting a higher specific activity than reported chondroitin ABC lyases. HH-chondroitin ABC lyase represents the first characterized chondroitin lyase from intestinal Firmicutes and offers a viable commercial option for the production of chondroitin, dermatan, and hyaluronan oligosaccharides and also for potential medical applications. IMPORTANCE An increased understanding of GAG metabolism by intestinal bacteria is critical in identifying the driving factors for the composition, modulation, and homeostasis of the human gut microbiota. In addition, GAG-depolymerizing polysaccharide lyases are highly desired enzymes for the production of GAG oligosaccharides and as therapeutics. At present, the dissection of the enzymatic machinery for GAG degradation is highly skewed toward Bacteroides. In this study, we have isolated an efficient GAG-degrading Firmicutes bacterium from human feces and characterized the first chondroitin ABC lyase from a Firmicutes, which complements the fundamental knowledge of GAG utilization in the human colon. The genomic and transcriptomic analysis of the bacterium shows that Firmicutes might use a distinct approach to catabolize GAGs from that used by Bacteroides. The high specific activity of the characterized chondroitin ABC lyase aids future attempts to develop a commercial chondroitinase for industrial and medicinal applications.
Keywords: Bacteroides; Firmicutes; Hungatella hathewayi; chondroitin ABC lyase; glycosaminoglycans.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

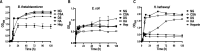





References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

