Adjuvant Abemaciclib Combined with Endocrine Therapy: Efficacy Results in monarchE Cohort 1
- PMID: 36342342
- PMCID: PMC9847542
- DOI: 10.1093/oncolo/oyac234
Adjuvant Abemaciclib Combined with Endocrine Therapy: Efficacy Results in monarchE Cohort 1
Abstract
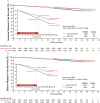
The monarchE Cohort 1 patient population was enrolled based on high-risk clinicopathological features that can easily be identified as part of routine clinical breast cancer evaluation. Efficacy data from Cohort 1 demonstrate substantial evidence of benefit for adjuvant abemaciclib+ET in patients with HR+, HER2- early breast cancer at high risk of recurrence (ClinicalTrials.gov: NCT03155997 [monarchE]).
Keywords: CDK4/6; Ki-67; abemaciclib; adjuvant; early breast cancer.
© The Author(s) 2022. Published by Oxford University Press.
Conflict of interest statement
Masakazu Toi reports grants and personal fees for research and honoraria from Chugai, Takeda, Pfizer, Kyowa-Kirin, Taiho, Eisai, Daiichi-Sankyo, AstraZeneca, Shimadzu, Yakult, and Nippon Kyaku; other fees for advisory role for drug development from Kyowa-Kirin and Daiichi-Sankyo; research grants from JBCRG association, Astellas, AFI Technologies, Shionogi, and GL Science; personal and other fees from Eli Lilly and Company for advisory role; personal fees for honoraria from MSD, Exact Science, and Novartis; personal fees and other for honoraria and advisory role from Konica Minolta, Bristol Myers Squibb; other fees for advisory role from Athenex Oncology, Bertis Terumo, and Kansai Medical Net; and serves as a board of director for JBCRG Association, Organisation for Oncology and Translational Research, and Kyoto Breast Cancer Research Network, outside the submitted work. Frances Boyle reports personal fees for honoraria and advisory role for Roche, Pfizer, Eli Lilly and Company, and Novartis outside the submitted work. Mattea Reinisch reports personal fees from AstraZeneca, MSD, Eli Lilly and Company, Roche, Daichi Sankyo, Seagen, and Somatex, as well as personal fees and non-financial support from Novartis and Pfizer, outside the submitted work. Ran Wei, Francisco Sapunar, Brenda R. Grimes, and Sarah Cassidy Nabinger are full-time employees of Eli Lilly and Company and/or Eli Lilly and company shareholders. Stephen R.D. Johnston reports personal fees and other from AstraZeneca, Eli Lilly and Company, Novartis, Pfizer, Eisai, and Roche/Genentech outside the submitted work. Young-Hyuck Im, David Molthrop, and Zefei Jiang indicated no financial relationships.
Figures
References
-
- Harbeck N, Rastogi P, Martin M, et al. . Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol. 2021;32(12):1571-1581. - PubMed
-
- VERZENIO™ (abemaciclib) Pharmaceuticals and Medical Devices Agency FY (April 2021-March 2022). 2021. Japan: Pharmaceuticals and Medical Devices Agency (PMDA).
-
- VERZENIOS™ (abemaciclib) European Medicines Agency. 2022. Amsterdam, Netherlands: European Medicines Agency.
-
- Rugo HS, O’Shaughnessy J, Boyle F, et al. . Adjuvant abemaciclib combined with endocrine therapy for high risk early breast cancer: safety and patient-reported outcomes from the monarchE study. Ann Oncol. 2022;33(6):616-627. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

