Vascular endothelial-cadherin as a marker of endothelial injury in preclinical Alzheimer disease
- PMID: 36342663
- PMCID: PMC9735377
- DOI: 10.1002/acn3.51685
Vascular endothelial-cadherin as a marker of endothelial injury in preclinical Alzheimer disease
Abstract
Objective: Endothelial dysfunction is an early and prevalent pathology in Alzheimer disease (AD). We here investigate the value of vascular endothelial-cadherin (VEC) as a cerebrospinal fluid (CSF) marker of endothelial injury in preclinical AD.
Methods: Cognitively normal participants (Clinical Dementia Rating [CDR] 0) from the Knight Washington University-ADRC were included in this study (n = 700). Preclinical Alzheimer's Cognitive Composite (PACC) scores, CSF VEC, tau, p-tau181, Aβ42/Aβ40, neurofilament light-chain (NFL) levels, and magnetic resonance imaging (MRI) assessments of white matter injury (WMI) were obtained from all participants. A subset of participants underwent brain amyloid imaging using positron emission tomography (amyloid-PET) (n = 534). Linear regression examined associations of CSF VEC with PACC and individual cognitive scores in preclinical AD. Mediation analyses examined whether CSF VEC mediated effects of CSF amyloid and tau markers on cognition in preclinical AD.
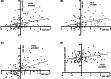
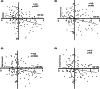
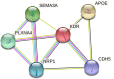
Results: CSF VEC levels significantly correlated with PACC and individual cognitive scores in participants with amyloid (A+T±N±; n = 558) or those with amyloid and tau pathologies (A+T+N±; n = 259), after adjusting for covariates. CSF VEC also correlated with CSF measures of amyloid, tau, and neurodegeneration and global amyloid burden on amyloid-PET scans in our cohort. Importantly, our findings suggest that CSF VEC mediates associations of CSF Aβ42/Aβ40, p-tau181, and global amyloid burden with cognitive outcomes in preclinical AD.
Interpretation: Our results support the utility of CSF VEC as a marker of endothelial injury in AD and highlight the importance of endothelial injury as an early pathology that contributes to cognitive impairment in even the earliest preclinical stages.
© 2022 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
CC has received research support from Biogen, Eisai, Alector, and Parabon. These funding agencies had no role in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. CC is a member of the advisory board of Vivid Genetics, Halia Therapeutics, and ADx Healthcare. JH is a paid consultant for Roche and Parabon Nanolabs. RT, RK, JS, and CLP have no conflict of interest.
Figures
References
-
- Price JL, Morris JC. Tangles and plaques in nondemented aging and "preclinical" Alzheimer's disease. Ann Neurol. 1999;45(3):358‐368. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
