Validation of SARS-CoV-2 pooled testing for surveillance using the Panther Fusion® system: Impact of pool size, automation, and assay chemistry
- PMID: 36342921
- PMCID: PMC9639840
- DOI: 10.1371/journal.pone.0276729
Validation of SARS-CoV-2 pooled testing for surveillance using the Panther Fusion® system: Impact of pool size, automation, and assay chemistry
Abstract
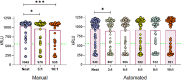
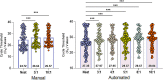
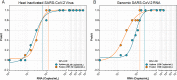
Combining diagnostic specimens into pools has been considered as a strategy to augment throughput, decrease turnaround time, and leverage resources. This study utilized a multi-parametric approach to assess optimum pool size, impact of automation, and effect of nucleic acid amplification chemistries on the detection of SARS-CoV-2 RNA in pooled samples for surveillance testing on the Hologic Panther Fusion® System. Dorfman pooled testing was conducted with previously tested SARS-CoV-2 nasopharyngeal samples using Hologic's Aptima® and Panther Fusion® SARS-CoV-2 Emergency Use Authorization assays. A manual workflow was used to generate pool sizes of 5:1 (five samples: one positive, four negative) and 10:1. An automated workflow was used to generate pool sizes of 3:1, 4:1, 5:1, 8:1 and 10:1. The impact of pool size, pooling method, and assay chemistry on sensitivity, specificity, and lower limit of detection (LLOD) was evaluated. Both the Hologic Aptima® and Panther Fusion® SARS-CoV-2 assays demonstrated >85% positive percent agreement between neat testing and pool sizes ≤5:1, satisfying FDA recommendation. Discordant results between neat and pooled testing were more frequent for positive samples with CT>35. Fusion® CT (cycle threshold) values for pooled samples increased as expected for pool sizes of 5:1 (CT increase of 1.92-2.41) and 10:1 (CT increase of 3.03-3.29). The Fusion® assay demonstrated lower LLOD than the Aptima® assay for pooled testing (956 vs 1503 cp/mL, pool size of 5:1). Lowering the cut-off threshold of the Aptima® assay from 560 kRLU (manufacturer's setting) to 350 kRLU improved the assay sensitivity to that of the Fusion® assay for pooled testing. Both Hologic's SARS-CoV-2 assays met the FDA recommended guidelines for percent positive agreement (>85%) for pool sizes ≤5:1. Automated pooling increased test throughput and enabled automated sample tracking while requiring less labor. The Fusion® SARS-CoV-2 assay, which demonstrated a lower LLOD, may be more appropriate for surveillance testing.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
The Hologic Aptima SARS-CoV-2 assay enables high ratio pooling saving reagents and improving turnaround time.J Clin Lab Anal. 2021 Sep;35(9):e23888. doi: 10.1002/jcla.23888. Epub 2021 Jul 2. J Clin Lab Anal. 2021. PMID: 34213803 Free PMC article.
-
Analytical and Clinical Comparison of Three Nucleic Acid Amplification Tests for SARS-CoV-2 Detection.J Clin Microbiol. 2020 Aug 24;58(9):e01134-20. doi: 10.1128/JCM.01134-20. Print 2020 Aug 24. J Clin Microbiol. 2020. PMID: 32571894 Free PMC article.
-
Comparison of a Point-of-Care Assay and a High-Complexity Assay for Detection of SARS-CoV-2 RNA.J Appl Lab Med. 2020 Nov 1;5(6):1307-1312. doi: 10.1093/jalm/jfaa135. J Appl Lab Med. 2020. PMID: 32761092 Free PMC article.
-
Strategies for Scaling up SARS-CoV-2 Molecular Testing Capacity.Clin Lab Med. 2022 Jun;42(2):261-282. doi: 10.1016/j.cll.2022.02.006. Epub 2022 Mar 8. Clin Lab Med. 2022. PMID: 35636826 Free PMC article. Review.
-
Effectiveness of Sample Pooling Strategies for SARS-CoV-2 Mass Screening by RT-PCR: A Scoping Review.J Lab Physicians. 2020 Dec;12(3):212-218. doi: 10.1055/s-0040-1721159. Epub 2020 Nov 23. J Lab Physicians. 2020. PMID: 33268939 Free PMC article.
References
-
- Reusken CBEM Broberg EK, Haagmans B, Meijer A, Corman VM, Papa A, et al.. Laboratory readiness and response for novel coronavirus (2019-nCoV) in expert laboratories in 30 EU/EEA countries, January 2020. Euro Surveill. 2020;25(6):2000082. Epub 02/11. doi: 10.2807/1560-7917.ES.2020.25.6.2000082 . - DOI - PMC - PubMed
-
- Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV) 2020. Available from: https://www.who.int/news/item/30-01-2020-statement-on-the-second-meeting....
-
- Mendoza RP, Bi C, Cheng HT, Gabutan E, Pagaspas GJ, Khan N, et al.. Implementation of a pooled surveillance testing program for asymptomatic SARS-CoV-2 infections in K-12 schools and universities. EClinicalMedicine. 2021;38:101028. Epub 2021/07/27. doi: 10.1016/j.eclinm.2021.101028 ; PubMed Central PMCID: PMC8286123. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

