Trans-regulatory changes underpin the evolution of the Drosophila immune response
- PMID: 36342922
- PMCID: PMC9671443
- DOI: 10.1371/journal.pgen.1010453
Trans-regulatory changes underpin the evolution of the Drosophila immune response
Abstract
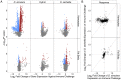
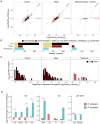
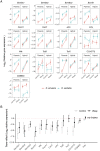
When an animal is infected, the expression of a large suite of genes is changed, resulting in an immune response that can defend the host. Despite much evidence that the sequence of proteins in the immune system can evolve rapidly, the evolution of gene expression is comparatively poorly understood. We therefore investigated the transcriptional response to parasitoid wasp infection in Drosophila simulans and D. sechellia. Although these species are closely related, there has been a large scale divergence in the expression of immune-responsive genes in their two main immune tissues, the fat body and hemocytes. Many genes, including those encoding molecules that directly kill pathogens, have cis regulatory changes, frequently resulting in large differences in their expression in the two species. However, these changes in cis regulation overwhelmingly affected gene expression in immune-challenged and uninfected animals alike. Divergence in the response to infection was controlled in trans. We argue that altering trans-regulatory factors, such as signalling pathways or immune modulators, may allow natural selection to alter the expression of large numbers of immune-responsive genes in a coordinated fashion.
Copyright: © 2022 Ding et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

