Autotoxin-mediated latecomer killing in yeast communities
- PMID: 36342925
- PMCID: PMC9639812
- DOI: 10.1371/journal.pbio.3001844
Autotoxin-mediated latecomer killing in yeast communities
Abstract
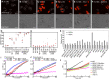
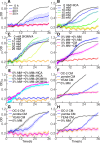
Cellular adaptation to stressful environments such as starvation is essential to the survival of microbial communities, but the uniform response of the cell community may lead to entire cell death or severe damage to their fitness. Here, we demonstrate an elaborate response of the yeast community against glucose depletion, in which the first adapted cells kill the latecomer cells. During glucose depletion, yeast cells release autotoxins, such as leucic acid and L-2keto-3methylvalerate, which can even kill the clonal cells of the ones producing them. Although these autotoxins were likely to induce mass suicide, some cells differentiated to adapt to the autotoxins without genetic changes. If nondifferentiated latecomers tried to invade the habitat, autotoxins damaged or killed the latecomers, but the differentiated cells could selectively survive. Phylogenetically distant fission and budding yeast shared this behavior using the same autotoxins, suggesting that latecomer killing may be the universal system of intercellular communication, which may be relevant to the evolutional transition from unicellular to multicellular organisms.
Copyright: © 2022 Oda et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: Patent applications have been filed for the technology described in this publication. A.H.O., T.S.H., and K.O. are named as the inventors of these patents. The remaining authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

