Risk and symptoms of COVID-19 in health professionals according to baseline immune status and booster vaccination during the Delta and Omicron waves in Switzerland-A multicentre cohort study
- PMID: 36342956
- PMCID: PMC9678290
- DOI: 10.1371/journal.pmed.1004125
Risk and symptoms of COVID-19 in health professionals according to baseline immune status and booster vaccination during the Delta and Omicron waves in Switzerland-A multicentre cohort study
Abstract
Background: Knowledge about protection conferred by previous Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection and/or vaccination against emerging viral variants allows clinicians, epidemiologists, and health authorities to predict and reduce the future Coronavirus Disease 2019 (COVID-19) burden. We investigated the risk and symptoms of SARS-CoV-2 (re)infection and vaccine breakthrough infection during the Delta and Omicron waves, depending on baseline immune status and subsequent vaccinations.
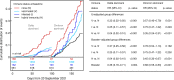
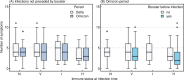
Methods and findings: In this prospective, multicentre cohort performed between August 2020 and March 2022, we recruited hospital employees from ten acute/nonacute healthcare networks in Eastern/Northern Switzerland. We determined immune status in September 2021 based on serology and previous SARS-CoV-2 infections/vaccinations: Group N (no immunity); Group V (twice vaccinated, uninfected); Group I (infected, unvaccinated); Group H (hybrid: infected and ≥1 vaccination). Date and symptoms of (re)infections and subsequent (booster) vaccinations were recorded until March 2022. We compared the time to positive SARS-CoV-2 swab and number of symptoms according to immune status, viral variant (i.e., Delta-dominant before December 27, 2021; Omicron-dominant on/after this date), and subsequent vaccinations, adjusting for exposure/behavior variables. Among 2,595 participants (median follow-up 171 days), we observed 764 (29%) (re)infections, thereof 591 during the Omicron period. Compared to group N, the hazard ratio (HR) for (re)infection was 0.33 (95% confidence interval [CI] 0.22 to 0.50, p < 0.001) for V, 0.25 (95% CI 0.11 to 0.57, p = 0.001) for I, and 0.04 (95% CI 0.02 to 0.10, p < 0.001) for H in the Delta period. HRs substantially increased during the Omicron period for all groups; in multivariable analyses, only belonging to group H was associated with protection (adjusted HR [aHR] 0.52, 95% CI 0.35 to 0.77, p = 0.001); booster vaccination was associated with reduction of breakthrough infection risk in groups V (aHR 0.68, 95% CI 0.54 to 0.85, p = 0.001) and H (aHR 0.67, 95% CI 0.45 to 1.00, p = 0.048), largely observed in the early Omicron period. Group H (versus N, risk ratio (RR) 0.80, 95% CI 0.66 to 0.97, p = 0.021) and participants with booster vaccination (versus nonboosted, RR 0.79, 95% CI 0.71 to 0.88, p < 0.001) reported less symptoms during infection. Important limitations are that SARS-CoV-2 swab results were self-reported and that results on viral variants were inferred from the predominating strain circulating in the community at that time, rather than sequencing.
Conclusions: Our data suggest that hybrid immunity and booster vaccination are associated with a reduced risk and reduced symptom number of SARS-CoV-2 infection during Delta- and Omicron-dominant periods. For previously noninfected individuals, booster vaccination might reduce the risk of symptomatic Omicron infection, although this benefit seems to wane over time.
Copyright: © 2022 Babouee Flury et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

