11B and 1H-11B HMBC NMR as a Tool for Identification of a Boron-Containing Nucleoside Dimer
- PMID: 36343012
- PMCID: PMC9707516
- DOI: 10.1021/acs.jnatprod.2c00745
11B and 1H-11B HMBC NMR as a Tool for Identification of a Boron-Containing Nucleoside Dimer
Abstract
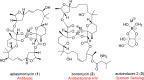
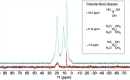
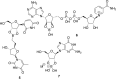
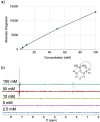
Boron-containing compounds are commonly used in synthetic chemistry and are known to play important roles in biology. Despite the widespread relevance of boronated compounds, there have been limited methods to discover, characterize, and study them. Here, we describe the use of 11B NMR, including 1H-11B HMBC, for the isolation and characterization of the boron-containing natural product diadenosine borate. Utilizing synthetic standards, we optimized coupling parameters for 1H-11B HMBC experiments to allow for the analysis of small quantities (∼1 mg) of boron-containing compounds. This work can facilitate the broader application of 11B NMR to the study of boron in a range of applications, from synthetic chemistry to the role of boron in naturally occurring systems.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Dembitsky V. M.; Gloriozova T. A. J. Nat. Prod. Resour. 2017, 3, 147–154.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

