Aβ and tau prions feature in the neuropathogenesis of Down syndrome
- PMID: 36343257
- PMCID: PMC9674250
- DOI: 10.1073/pnas.2212954119
Aβ and tau prions feature in the neuropathogenesis of Down syndrome
Abstract
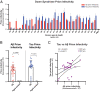
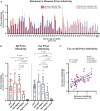
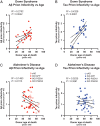
Down syndrome (DS) is caused by the triplication of chromosome 21 and is the most common chromosomal disorder in humans. Those individuals with DS who live beyond age 40 y develop a progressive dementia that is similar to Alzheimer's disease (AD). Both DS and AD brains exhibit numerous extracellular amyloid plaques composed of Aβ and intracellular neurofibrillary tangles composed of tau. Since AD is a double-prion disorder, we asked if both Aβ and tau prions feature in DS. Frozen brains from people with DS, familial AD (fAD), sporadic AD (sAD), and age-matched controls were procured from brain biorepositories. We selectively precipitated Aβ and tau prions from DS brain homogenates and measured the number of prions using cellular bioassays. In brain extracts from 28 deceased donors with DS, ranging in age from 19 to 65 y, we found nearly all DS brains had readily measurable levels of Aβ and tau prions. In a cross-sectional analysis of DS donor age at death, we found that the levels of Aβ and tau prions increased with age. In contrast to DS brains, the levels of Aβ and tau prions in the brains of 37 fAD and sAD donors decreased as a function of age at death. Whether DS is an ideal model for assessing the efficacy of putative AD therapeutics remains to be determined.
Keywords: Aβ; Down syndrome; cellular bioassays; prions; tau.
Conflict of interest statement
Competing Interest Statement: S.B.P. is the founder of Prio-Pharma, which did not contribute financial or any other support to these studies. The opinions and assertions expressed herein are those of the authors and do not reflect the official policy or position of the Uniformed Services University of the Health Sciences or the Department of Defense.
Figures
References
-
- Alzheimer A., Ueber eine eigenartige Erkrankung der Hirnrinde. Cent. Nervenheilk. Psychiat. 30, 177–179 (1907).
-
- Vos T., et al. ; GBD 2015 Disease and Injury Incidence and Prevalence Collaborators, Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1545–1602 (2016). - PMC - PubMed
-
- Lejeune J., Gautier M., Turpin R., [Study of somatic chromosomes from 9 mongoloid children]. C. R. Hebd. Seances Acad. Sci. 248, 1721–1722 (1959). - PubMed
-
- Chapman R. S., Hesketh L. J., Behavioral phenotype of individuals with Down syndrome. Ment. Retard. Dev. Disabil. Res. Rev. 6, 84–95 (2000). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 AG023501/AG/NIA NIH HHS/United States
- P01 AG002132/AG/NIA NIH HHS/United States
- P30 AG066519/AG/NIA NIH HHS/United States
- P30 AG062422/AG/NIA NIH HHS/United States
- P01 AG019724/AG/NIA NIH HHS/United States
- MRC_/Medical Research Council/United Kingdom
- P30 AG066509/AG/NIA NIH HHS/United States
- U01 AG006781/AG/NIA NIH HHS/United States
- P30AG066509/UW Alzheimer's Disease Research Center
- P01AG002132/HHS | National Institutes of Health (NIH)
- P30AG066519/HHS | NIH | National Institute on Aging (NIA)
- U01AG006781/Adult Changes in Thought Study

