High-density spinal cord stimulation selectively activates lower urinary tract nerves
- PMID: 36343359
- PMCID: PMC9855651
- DOI: 10.1088/1741-2552/aca0c2
High-density spinal cord stimulation selectively activates lower urinary tract nerves
Abstract
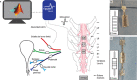
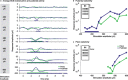
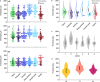
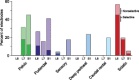
Objective.Epidural spinal cord stimulation (SCS) is a potential intervention to improve limb and autonomic functions, with lumbar stimulation improving locomotion and thoracic stimulation regulating blood pressure. Here, we asked whether sacral SCS could be used to target the lower urinary tract (LUT) and used a high-density epidural electrode array to test whether individual electrodes could selectively recruit LUT nerves.Approach. We placed a high-density epidural SCS array on the dorsal surface of the sacral spinal cord and cauda equina of anesthetized cats and recorded the stimulation-evoked activity from nerve cuffs on the pelvic, pudendal and sciatic nerves.Main results. Here we show that sacral SCS evokes responses in nerves innervating the bladder and urethra and that these nerves can be activated selectively. Sacral SCS always recruited the pelvic and pudendal nerves and selectively recruited both of these nerves in all but one animal. Individual branches of the pudendal nerve were always recruited as well. Electrodes that selectively recruited specific peripheral nerves were spatially clustered on the arrays, suggesting anatomically organized sensory pathways.Significance.This selective recruitment demonstrates a mechanism to directly modulate bladder and urethral function through known reflex pathways, which could be used to restore bladder and urethral function after injury or disease.
Keywords: autonomic nervous system; bladder; neuromodulation; peripheral nervous system; spinal cord stimulation.
Creative Commons Attribution license.
Figures


Similar articles
-
A Computational Study of Lower Urinary Tract Nerve Recruitment with Epidural Stimulation of the Lumbosacral Spinal Cord.Annu Int Conf IEEE Eng Med Biol Soc. 2022 Jul;2022:744-747. doi: 10.1109/EMBC48229.2022.9871292. Annu Int Conf IEEE Eng Med Biol Soc. 2022. PMID: 36086335
-
Intraurethral stimulation evokes bladder responses via 2 distinct reflex pathways.J Urol. 2009 Jul;182(1):366-73. doi: 10.1016/j.juro.2009.02.110. Epub 2009 May 17. J Urol. 2009. PMID: 19447414 Free PMC article.
-
Multiple pudendal sensory pathways reflexly modulate bladder and urethral activity in patients with spinal cord injury.J Urol. 2011 Feb;185(2):737-43. doi: 10.1016/j.juro.2010.09.079. Epub 2010 Dec 18. J Urol. 2011. PMID: 21168860 Free PMC article.
-
Urinary bladder control by electrical stimulation: review of electrical stimulation techniques in spinal cord injury.Neurourol Urodyn. 1997;16(1):39-53. doi: 10.1002/(sici)1520-6777(1997)16:1<39::aid-nau6>3.0.co;2-f. Neurourol Urodyn. 1997. PMID: 9021789 Review.
-
The mechanisms of electrical neuromodulation.J Physiol. 2025 Jan;603(2):247-284. doi: 10.1113/JP286205. Epub 2024 Dec 30. J Physiol. 2025. PMID: 39740777 Review.
Cited by
-
The inhibitory effect of intraspinal microstimulation of the sacral spinal cord on nonlinear bladder reflex dynamics in cats.Front Neurosci. 2025 Feb 3;19:1519377. doi: 10.3389/fnins.2025.1519377. eCollection 2025. Front Neurosci. 2025. PMID: 39963259 Free PMC article.
-
Nerve transfer for restoration of lower motor neuron-lesioned bladder, urethral and anal sphincter function. Part 4: Effectiveness of the motor reinnervation.Am J Physiol Regul Integr Comp Physiol. 2024 Jun 1;326(6):R528-R551. doi: 10.1152/ajpregu.00248.2023. Epub 2024 Mar 18. Am J Physiol Regul Integr Comp Physiol. 2024. PMID: 38497126 Free PMC article.
-
Towards Precise Synthetic Neural Codes: High-dimensional Stimulation with Flexible Electrodes.Npj Flex Electron. 2025;9(1):68. doi: 10.1038/s41528-025-00447-y. Epub 2025 Jul 14. Npj Flex Electron. 2025. PMID: 40718756 Free PMC article.
-
Selective Activation of the Spinal Cord with Epidural Electrical Stimulation.Brain Sci. 2024 Jun 27;14(7):650. doi: 10.3390/brainsci14070650. Brain Sci. 2024. PMID: 39061391 Free PMC article.
References
-
- Brown J, McGhan W, Chokroverty S. Comorbidities associated with overactive bladder. Am. J. Manage. Care. 2000;6:S574–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
