Current approaches to malaria vaccines
- PMID: 36343566
- PMCID: PMC11127243
- DOI: 10.1016/j.mib.2022.102227
Current approaches to malaria vaccines
Abstract
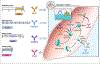
The complex Plasmodium life cycle offers different vaccine approaches with distinct parasitological and clinical effects. The approaches and their rationales were established decades ago: vaccines targeting pre-erythrocytic (sporozoite and liver-stage) parasites prevent infection, those to blood-stage parasites reduce disease, and those to sexual-stage parasites or mosquito vector reduce transmission and eliminate malaria through herd immunity. The pre-erythrocytic RTS,S vaccine (Mosquirix, GlaskoSmithKline (GSK)), recommended by WHO in 2021, reduces clinical malaria in children. Knowledge of parasite biology, host-parasite interactions, and immune mechanisms is informing new concepts to improve on RTS,S and to target other parasite stages. This review emphasizes vaccine approaches and candidates currently in the clinic or likely to enter clinical testing soon.
Copyright © 2022. Published by Elsevier Ltd.
Conflict of interest statement
Conflict of interest statement
The authors report no conflict of interest.
Figures


References
-
- Moon JE, Ockenhouse C, Regules JA, Vekemans J, Lee C, Chuang I, Traskine M, Jongert E, Ivinson K, Morelle D, et al. : A phase IIa controlled human malaria infection and immunogenicity study of RTS,S/AS01E and RTS,S/AS01B delayed fractional dose regimens in Malaria-Naive adults. J Infect Dis 2020, 222:1681–1691. - PMC - PubMed
-
-
Chandramohan D, Zongo I, Sagara I, Cairns M, Yerbanga RS, Diarra M, Nikiema F, Tapily A, Sompougdou F, Issiaka D, et al. : Seasonal malaria vaccination with or without seasonal malaria chemoprevention. N Engl J Med 2021, 385:1005–1017.
•• Phase 3 study showing unprecedented efficacy with RTS,S vaccine when used in conjunction with Seasonal Malaria Chemoprevention (monthly antimalarials during rainy season) and timed to maximize titers during transmission period.
-
-
-
Datoo MS, Natama MH, Some A, Traore O, Rouamba T, Bellamy D, Yameogo P, Valia D, Tegneri M, Ouedraogo F, et al. : Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso: a randomised controlled trial. Lancet 2021, 397:1809–1818.
• Phase 2 trial of RTS,S-like vaccine R21 in Matrix M™ formulation reported > 70% efficacy against clinical malaria in Burkinabe children, justifying advancement to Phase 3 testing.
-
-
- Clyde DF, Most H, McCarthy VC, Vanderberg JP: Immunization of man against sporozite-induced falciparum malaria. Am J Med Sci 1973, 266:169–177. - PubMed
-
- Clyde DF: Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites. Am J Trop Med Hyg 1975, 24:397–401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

