Pharmacokinetics and efficacy of topical inserts containing tenofovir alafenamide fumarate and elvitegravir administered rectally in macaques
- PMID: 36343572
- PMCID: PMC9643401
- DOI: 10.1016/j.ebiom.2022.104338
Pharmacokinetics and efficacy of topical inserts containing tenofovir alafenamide fumarate and elvitegravir administered rectally in macaques
Abstract
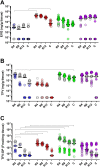
Background: Topical on-demand forms for HIV pre-exposure prophylaxis (PrEP) may be a desirable alternative for people that prefer not to use daily PrEP. CONRAD has developed inserts containing tenofovir alafenamide (TAF) and elvitegravir (EVG) for on-demand vaginal or rectal pericoital use. We assessed the pharmacokinetics (PK) and pre-exposure efficacy of rectally applied TAF/EVG inserts in macaques.
Methods: PK was assessed in 12 pigtailed macaques. Tenofovir (TFV) and EVG levels were assayed in rectal biopsies and secretions, and tenofovir-diphosphate (TFV-DP) levels in biopsies and peripheral blood mononuclear cells (PBMC). Drug biodistribution was evaluated in 10 animals at necropsy 4 h post-dosing. For efficacy assessments, one or two TAF/EVG inserts were administered to macaques (n = 6) 4 h before repeated rectal SHIV162p3 challenges.
Findings: One TAF/EVG insert resulted in rapid and high EVG and TFV-DP in rectal tissue 4 h after application. Adding a second insert led to a 10-fold increase in EVG and TFV-DP in rectal tissue. Efficacy of one and two TAF/EVG inserts were 72.6% (CI 24.5%-92.6%) and 93.1% (CI 73.3%-99.2%), respectively.
Interpretation: Although high TFV-DP and EVG levels were observed with one rectal TAF/EVG insert, it only conferred partial protection from rectal SHIV challenges. Adding a second insert led to an increase in TFV and EVG in rectal tissues resulting in higher (>90%) efficacy. These results highlight the high efficacy of TAF/EVG inserts as topical on-demand rectal PrEP, as well as the need for appropriate drug coverage in the deep rectum and colon to achieve high protection.
Funding: The work related to animal studies was funded by CDC intramural funds and an interagency agreement between CDC and USAID (USAID/CDC IAA AID-GH-T-15-00002). The work related to the insert formulation was funded by U.S. PEPFAR through USAID under a Cooperative Agreement (AID-OAA-A-14-00010) with CONRAD/Eastern Virginia Medical School. The findings and conclusions of this manuscript are those of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention (CDC), USAID, President's Emergency Plan for AIDS Relief (PEPFAR), Eastern Virginia Medical School (EVMS), or the US government.
Keywords: Elvitegravir; HIV pre-exposure prophylaxis; Macaque models; Tenofovir alafenamide; Topical PrEP.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests J.G.G-L and W.H. are named in the US. Government (USG) patents on “Inhibition of HIV infection through chemoprophylaxis” and “HIV post-exposure prophylaxis” and a patent application on “HIV pre-exposure prophylaxis”. W. H. and J. G. G.-L. report royalties or licenses from Mylan, Laurus Generics, TAD Pharma, and CIPLA Limited. M.M.P., V.A., G.F.D., and M.R.C. are named in patent applications on “Pharmaceutical compositions and methods of making on-demand solid dosage formulations,” inventions that were developed under US Agency for International Development (USAID)-funded cooperative agreement. N.M., T.S., J. M., A.H., C.D., M.M., Y.P., and J.M.S., declare no competing interest. The findings and conclusions of this manuscript are those of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention (CDC), USAID, President's Emergency Plan for AIDS Relief (PEPFAR), Eastern Virginia Medical School (EVMS), or the USG.
Figures



References
-
- CDC. Centers for Disease Control and Prevention . Vol. 32. 2021. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html (HIV surveillance report, 2019). Available from:
-
- Riddell Jt, Amico K.R., Mayer K.H. HIV preexposure prophylaxis: a review. JAMA. 2018;319(12):1261–1268. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

