Targeting caspase-2 interactions with tau in Alzheimer's disease and related dementias
- PMID: 36343883
- PMCID: PMC9991976
- DOI: 10.1016/j.trsl.2022.10.009
Targeting caspase-2 interactions with tau in Alzheimer's disease and related dementias
Abstract
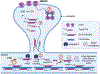
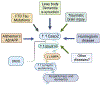
Targeting amyloid-β plaques and tau tangles has failed to provide effective treatments for Alzheimer's disease and related dementias (ADRD). A more fruitful pathway to ADRD therapeutics may be the development of therapies that target common signaling pathways that disrupt synaptic connections and impede communication between neurons. In this review, we present our characterization of a signaling pathway common to several neurological diseases featuring dementia including Alzheimer's disease, frontotemporal dementia, Lewy body dementia, and Huntington's disease. This signaling pathway features the cleavage of tau by caspase-2 (Casp2) yielding Δtau314 (Casp2/tau/Δtau314). Through a not yet fully delineated mechanism, Δtau314 catalyzes the mislocalization and accumulation of tau to dendritic spines leading to the internalization of AMPA receptors and the concomitant weakening of synaptic transmission. Here, we review the accumulated evidence supporting Casp2 as a druggable target and its importance in ADRD. Additionally, we provide a brief overview of our initial medicinal chemistry explorations aimed at the preparation of novel, brain penetrant Casp2 inhibitors. We anticipate that this review will spark broader interest in Casp2 as a target for restoring synaptic dysfunction in ADRD.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
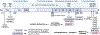
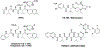
References
-
- Miles MA, Kitevska-Ilioski T, Hawkins CJ. Old and novel functions of caspase-2. International review of cell and molecular biology. 2017;332:155–212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

