The Impact of Highly Effective Modulator Therapy on Cystic Fibrosis Microbiology and Inflammation
- PMID: 36344072
- PMCID: PMC10224747
- DOI: 10.1016/j.ccm.2022.06.007
The Impact of Highly Effective Modulator Therapy on Cystic Fibrosis Microbiology and Inflammation
Abstract
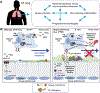
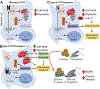
Highly effective cystic fibrosis (CF) transmembrane conductance regulator (CFTR) modulator therapy (HEMT) corrects the underlying molecular defect causing CF disease. HEMT decreases symptom burden and improves clinical metrics and quality of life for most people with CF (PwCF) and eligible cftr mutations. Improvements in measures of pulmonary health suggest that restoration of function of defective CFTR anion channels by HEMT not only enhances airway mucociliary clearance, but also reduces chronic pulmonary infection and inflammation. This article reviews the evidence for how HEMT influences the dynamic and interdependent processes of infection and inflammation in the CF airway, and what questions remain unanswered.
Keywords: Cystic fibrosis; Cystic fibrosis transmembrane regulator; Highly effective cystic fibrosis transmembrane conductance regulator modulator therapy (HEMT); Infection; Inflammation; Modulator.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure None of the authors has any commercial or financial conflicts of interest. L.J. Caverly receives funding from the National Institutes of Health (NIH) (K23HL136934) and the Cystic Fibrosis Foundation (CFF) (CAVERL20Y5). S.A. Riquelme receives funding from Vertex Research Innovation Award (PG010094), the CFF (RIQUEL21I0), and NIH (1R35HL135800). K.B. Hisert receives funding from the NIH (K08 HL136786) and the CFF (HISERT20A0 and HISERT19R3).
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

