Loss of NF1 in Drosophila Larvae Causes Tactile Hypersensitivity and Impaired Synaptic Transmission at the Neuromuscular Junction
- PMID: 36344265
- PMCID: PMC9794380
- DOI: 10.1523/JNEUROSCI.0562-22.2022
Loss of NF1 in Drosophila Larvae Causes Tactile Hypersensitivity and Impaired Synaptic Transmission at the Neuromuscular Junction
Abstract
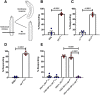
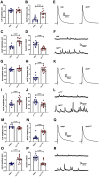
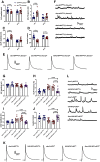
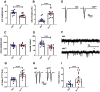
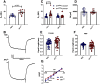
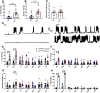
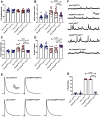
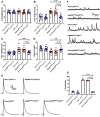
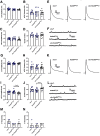
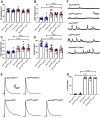
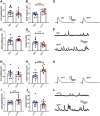
Autism spectrum disorder (ASD) is a neurodevelopmental condition in which the mechanisms underlying its core symptomatology are largely unknown. Studying animal models of monogenic syndromes associated with ASD, such as neurofibromatosis type 1 (NF1), can offer insights into its etiology. Here, we show that loss of function of the Drosophila NF1 ortholog results in tactile hypersensitivity following brief mechanical stimulation in the larva (mixed sexes), paralleling the sensory abnormalities observed in individuals with ASD. Mutant larvae also exhibit synaptic transmission deficits at the glutamatergic neuromuscular junction (NMJ), with increased spontaneous but reduced evoked release. While the latter is homeostatically compensated for by a postsynaptic increase in input resistance, the former is consistent with neuronal hyperexcitability. Indeed, diminished expression of NF1 specifically within central cholinergic neurons induces both excessive neuronal firing and tactile hypersensitivity, suggesting the two may be linked. Furthermore, both impaired synaptic transmission and behavioral deficits are fully rescued via knock-down of Ras proteins. These findings validate NF1 -/- Drosophila as a tractable model of ASD with the potential to elucidate important pathophysiological mechanisms.SIGNIFICANCE STATEMENT Autism spectrum disorder (ASD) affects 1-2% of the overall population and can considerably impact an individual's quality of life. However, there are currently no treatments available for its core symptoms, largely because of a poor understanding of the underlying mechanisms involved. Examining how loss of function of the ASD-associated NF1 gene affects behavior and physiology in Drosophila may shed light on this. In this study, we identify a novel, ASD-relevant behavioral phenotype in NF1 -/- larvae, namely an enhanced response to mechanical stimulation, which is associated with Ras-dependent synaptic transmission deficits indicative of neuronal hyperexcitability. Such insights support the use of Drosophila neurofibromatosis type 1 (NF1) models in ASD research and may provide outputs for genetic or pharmacological screens in future studies.
Keywords: Drosophila; NF1; Ras; autism spectrum disorder; neuromuscular junction; synaptic transmission.
Copyright © 2022 the authors.
Figures

Similar articles
-
A Targeted, Low-Throughput Compound Screen in a Drosophila Model of Neurofibromatosis Type 1 Identifies Simvastatin and BMS-204352 as Potential Therapies for Autism Spectrum Disorder (ASD).eNeuro. 2023 May 16;10(5):ENEURO.0461-22.2023. doi: 10.1523/ENEURO.0461-22.2023. Print 2023 May. eNeuro. 2023. PMID: 37185294 Free PMC article.
-
The Putative Drosophila TMEM184B Ortholog Tmep Ensures Proper Locomotion by Restraining Ectopic Firing at the Neuromuscular Junction.Mol Neurobiol. 2022 Apr;59(4):2605-2619. doi: 10.1007/s12035-022-02760-3. Epub 2022 Feb 2. Mol Neurobiol. 2022. PMID: 35107803 Free PMC article.
-
A Novel Human CAMK2A Mutation Disrupts Dendritic Morphology and Synaptic Transmission, and Causes ASD-Related Behaviors.J Neurosci. 2017 Feb 22;37(8):2216-2233. doi: 10.1523/JNEUROSCI.2068-16.2017. Epub 2017 Jan 27. J Neurosci. 2017. PMID: 28130356 Free PMC article.
-
An executive functioning perspective in neurofibromatosis type 1: from ADHD and autism spectrum disorder to research domains.Childs Nerv Syst. 2020 Oct;36(10):2321-2332. doi: 10.1007/s00381-020-04745-w. Epub 2020 Jul 3. Childs Nerv Syst. 2020. PMID: 32617712 Review.
-
Electrophysiological analysis of synaptic transmission in Drosophila.Wiley Interdiscip Rev Dev Biol. 2017 Sep;6(5):10.1002/wdev.277. doi: 10.1002/wdev.277. Epub 2017 May 24. Wiley Interdiscip Rev Dev Biol. 2017. PMID: 28544556 Free PMC article. Review.
Cited by
-
Neurofibromin deficiency alters the patterning and prioritization of motor behaviors in a state-dependent manner.bioRxiv [Preprint]. 2024 Aug 9:2024.08.08.607070. doi: 10.1101/2024.08.08.607070. bioRxiv. 2024. Update in: J Neurosci. 2025 Apr 16;45(16):e1531242025. doi: 10.1523/JNEUROSCI.1531-24.2025. PMID: 39149363 Free PMC article. Updated. Preprint.
-
Unraveling neuronal and metabolic alterations in neurofibromatosis type 1.J Neurodev Disord. 2024 Aug 31;16(1):49. doi: 10.1186/s11689-024-09565-6. J Neurodev Disord. 2024. PMID: 39217323 Free PMC article. Review.
-
Modeling neurodegenerative and neurodevelopmental disorders in the Drosophila mushroom body.Learn Mem. 2024 Jun 14;31(5):a053816. doi: 10.1101/lm.053816.123. Print 2024 May. Learn Mem. 2024. PMID: 38876485 Free PMC article. Review.
-
A Targeted, Low-Throughput Compound Screen in a Drosophila Model of Neurofibromatosis Type 1 Identifies Simvastatin and BMS-204352 as Potential Therapies for Autism Spectrum Disorder (ASD).eNeuro. 2023 May 16;10(5):ENEURO.0461-22.2023. doi: 10.1523/ENEURO.0461-22.2023. Print 2023 May. eNeuro. 2023. PMID: 37185294 Free PMC article.
-
Neurofibromin 1 regulates early developmental sleep in Drosophila.Neurobiol Sleep Circadian Rhythms. 2023 Aug 9;15:100101. doi: 10.1016/j.nbscr.2023.100101. eCollection 2023 Nov. Neurobiol Sleep Circadian Rhythms. 2023. PMID: 37593040 Free PMC article.
References
-
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, Ed 5. Arlington: American Psychiatric Association.
-
- Begum-Ali J, Kolesnik-Taylor A, Quiroz I, Mason L, Garg S, Green J, Johnson MH, Jones EJH; STAARS and EDEN Teams (2021) Early differences in auditory processing relate to autism spectrum disorder traits in infants with neurofibromatosis type I. J Neurodev Disord 13:22. 10.1186/s11689-021-09364-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
