Unlocking the potential of agonist antibodies for treating cancer using antibody engineering
- PMID: 36344331
- PMCID: PMC9742327
- DOI: 10.1016/j.molmed.2022.09.012
Unlocking the potential of agonist antibodies for treating cancer using antibody engineering
Abstract
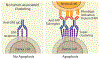
Agonist antibodies that target immune checkpoints, such as those in the tumor necrosis factor receptor (TNFR) superfamily, are an important class of emerging therapeutics due to their ability to regulate immune cell activity, especially for treating cancer. Despite their potential, to date, they have shown limited clinical utility and further antibody optimization is urgently needed to improve their therapeutic potential. Here, we discuss key antibody engineering approaches for improving the activity of antibody agonists by optimizing their valency, specificity for different receptors (e.g., bispecific antibodies) and epitopes (e.g., biepitopic or biparatopic antibodies), and Fc affinity for Fcγ receptors (FcγRs). These powerful approaches are being used to develop the next generation of cancer immunotherapeutics with improved efficacy and safety.
Keywords: 4-1BB; CD137; CD40; OX40; immunotherapy; monoclonal antibody.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests No interests are declared.
Figures

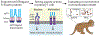
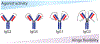
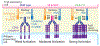

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

