Social interactions lead to motility-induced phase separation in fire ants
- PMID: 36344501
- PMCID: PMC9640710
- DOI: 10.1038/s41467-022-34181-0
Social interactions lead to motility-induced phase separation in fire ants
Abstract
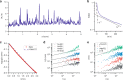
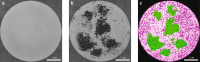
Collections of fire ants are a form of active matter, as the ants use their internal metabolism to self-propel. In the absence of aligning interactions, theory and simulations predict that active matter with spatially dependent motility can undergo motility-induced phase separation. However, so far in experiments, the motility effects that drive this process have come from either crowding or an external parameter. Though fire ants are social insects that communicate and cooperate in nontrivial ways, we show that the effect of their interactions can also be understood within the framework of motility-induced phase separation. In this context, the slowing down of ants when they approach each other results in an effective attraction that can lead to space-filling clusters and an eventual formation of dynamical heterogeneities. These results illustrate that motility-induced phase separation can provide a unifying framework to rationalize the behavior of a wide variety of active matter systems.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Effects of sea-level rise on physiological ecology of populations of a ground-dwelling ant.PLoS One. 2020 Apr 17;15(4):e0223304. doi: 10.1371/journal.pone.0223304. eCollection 2020. PLoS One. 2020. PMID: 32302310 Free PMC article.
-
Fire ant envenomation in children.Pediatrics. 1984 May;73(5):689-92. Pediatrics. 1984. PMID: 6201811
-
Pain and Lethality Induced by Insect Stings: An Exploratory and Correlational Study.Toxins (Basel). 2019 Jul 21;11(7):427. doi: 10.3390/toxins11070427. Toxins (Basel). 2019. PMID: 31330893 Free PMC article.
-
Allergy to imported fire ants.South Med J. 1989 Dec;82(12):1520-7. doi: 10.1097/00007611-198912000-00015. South Med J. 1989. PMID: 2688130 Review. No abstract available.
-
Global View on Ant Venom Allergy: from Allergenic Components to Clinical Management.Clin Rev Allergy Immunol. 2022 Feb;62(1):123-144. doi: 10.1007/s12016-021-08858-1. Epub 2021 Jun 1. Clin Rev Allergy Immunol. 2022. PMID: 34075569 Review.
Cited by
-
Entropy by Neighbor Distance as a New Measure for Characterizing Spatiotemporal Orders in Microscopic Collective Systems.Micromachines (Basel). 2023 Jul 26;14(8):1503. doi: 10.3390/mi14081503. Micromachines (Basel). 2023. PMID: 37630039 Free PMC article.
-
Synchronous and Fully Steerable Active Particle Systems for Enhanced Mimicking of Collective Motion in Nature.Adv Mater. 2024 Feb;36(7):e2304759. doi: 10.1002/adma.202304759. Epub 2023 Dec 4. Adv Mater. 2024. PMID: 37572374 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

