Microfluidics-integrated spaceflight hardware for measuring muscle strength of Caenorhabditis elegans on the International Space Station
- PMID: 36344513
- PMCID: PMC9640571
- DOI: 10.1038/s41526-022-00241-4
Microfluidics-integrated spaceflight hardware for measuring muscle strength of Caenorhabditis elegans on the International Space Station
Abstract
Caenorhabditis elegans is a low-cost genetic model that has been flown to the International Space Station to investigate the influence of microgravity on changes in the expression of genes involved in muscle maintenance. These studies showed that genes that encode muscle attachment complexes have decreased expression under microgravity. However, it remains to be answered whether the decreased expression leads to concomitant changes in animal muscle strength, specifically across multiple generations. We recently reported the NemaFlex microfluidic device for the measurement of muscle strength of C. elegans (Rahman et al., Lab Chip, 2018). In this study, we redesign our original NemaFlex device and integrate it with flow control hardware for spaceflight investigations considering mixed animal culture, constraints on astronaut time, crew safety, and on-orbit operations. The technical advances we have made include (i) a microfluidic device design that allows animals of a given size to be sorted from unsynchronized cultures and housed in individual chambers, (ii) a fluid handling protocol for injecting the suspension of animals into the microfluidic device that prevents channel clogging, introduction of bubbles, and crowding of animals in the chambers, and (iii) a custom-built worm-loading apparatus interfaced with the microfluidic device that allows easy manipulation of the worm suspension and prevents fluid leakage into the surrounding environment. Collectively, these technical advances enabled the development of new microfluidics-integrated hardware for spaceflight studies in C. elegans. Finally, we report Earth-based validation studies to test this new hardware, which has led to it being flown to the International Space Station.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests. S.A.V. and M.R. are co-founders of NemaLife Inc., which has licensed the microfluidic technology for commercialization.
Figures

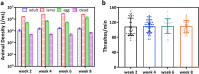



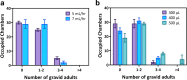
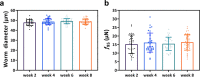
Similar articles
-
Spaceflight Induces Strength Decline in Caenorhabditis elegans.Cells. 2023 Oct 17;12(20):2470. doi: 10.3390/cells12202470. Cells. 2023. PMID: 37887314 Free PMC article.
-
WormSpace μ-TAS enabling automated on-chip multi-strain culturing and multi-function imaging of Caenorhabditis elegans at the single-worm level on the China Space Station.Lab Chip. 2024 Jul 10;24(14):3388-3402. doi: 10.1039/d4lc00210e. Lab Chip. 2024. PMID: 38818738
-
Microgravity alters the expressions of DNA repair genes and their regulatory miRNAs in space-flown Caenorhabditis elegans.Life Sci Space Res (Amst). 2023 May;37:25-38. doi: 10.1016/j.lssr.2023.02.002. Epub 2023 Feb 17. Life Sci Space Res (Amst). 2023. PMID: 37087176
-
Studying Parkinson's disease using Caenorhabditis elegans models in microfluidic devices.Integr Biol (Camb). 2019 May 1;11(5):186-207. doi: 10.1093/intbio/zyz017. Integr Biol (Camb). 2019. PMID: 31251339 Review.
-
Spaceflight bioreactor studies of cells and tissues.Adv Space Biol Med. 2002;8:177-95. doi: 10.1016/s1569-2574(02)08019-x. Adv Space Biol Med. 2002. PMID: 12951697 Review.
Cited by
-
A Compact Imaging Platform for Conducting C. elegans Phenotypic Assays on Earth and in Spaceflight.Life (Basel). 2023 Jan 10;13(1):200. doi: 10.3390/life13010200. Life (Basel). 2023. PMID: 36676149 Free PMC article.
-
Spaceflight Induces Strength Decline in Caenorhabditis elegans.Cells. 2023 Oct 17;12(20):2470. doi: 10.3390/cells12202470. Cells. 2023. PMID: 37887314 Free PMC article.
-
Advancing insights into microgravity induced muscle changes using Caenorhabditis elegans as a model organism.NPJ Microgravity. 2024 Jul 26;10(1):79. doi: 10.1038/s41526-024-00418-z. NPJ Microgravity. 2024. PMID: 39060303 Free PMC article. Review.
-
Lab-on-chip technologies for space research - current trends and prospects.Mikrochim Acta. 2023 Dec 14;191(1):31. doi: 10.1007/s00604-023-06084-4. Mikrochim Acta. 2023. PMID: 38095809 Free PMC article. Review.
References
-
- Vandenburgh H, Chromiak J, Shansky J, Del Tatto M, Lemaire J. Space travel directly induces skeletal muscle atrophy. FASEB J. 1999;13:1031–1038. - PubMed
-
- Trappe S, et al. Exercise in space: human skeletal muscle after 6 months aboard the International Space Station. J. Appl Physiol. 2009;106:1159–1168. - PubMed
-
- Booth FW. Molecular events underlying skeletal muscle atrophy and the development of effective countermeasures. Int. J. Sports Med. 1997;18:S265–9. - PubMed
-
- di Prampero PE, Narici MV. Muscles in microgravity: from fibres to human motion. J. Biomech. 2003;36:403–412. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

