Neuroprotective action of α-Klotho against LPS-activated glia conditioned medium in primary neuronal culture
- PMID: 36344527
- PMCID: PMC9640694
- DOI: 10.1038/s41598-022-21132-4
Neuroprotective action of α-Klotho against LPS-activated glia conditioned medium in primary neuronal culture
Abstract
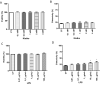
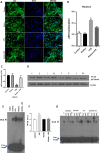
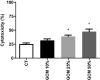
The α-Klotho is an anti-aging protein that, when overexpressed, extends the life span in humans and mice. It has an anti-inflammatory and protective action on renal cells by inhibiting NF-κB activation and production of inflammatory cytokines in response to TNF-α. Furthermore, studies have shown the neuroprotective effect of α-Klotho against neuroinflammation on different conditions, such as aging, animal models of neurodegenerative diseases, and ischemic brain injury. This work aimed to evaluate the effects of α-Klotho protein on primary glial cell culture against the proinflammatory challenge with LPS and how this could interfere with neuronal health. Cortical mixed glial cells and purified astrocytes were pretreated with α- α-Klotho and stimulated with LPS followed by TNFα, IL-1β, IL-6, IFN-γ levels, and NF-κB activity analysis. Conditioned medium from cortical mixed glia culture treated with LPS (glia conditioned medium (GCM) was used to induce neuronal death of primary cortical neuronal culture and evaluate if GCM-KL (medium from glia culture pretreated α-Klotho followed by LPS stimulation) or GCM + LPS in the presence of KL can reverse the effect. LPS treatment in glial cells induced an increase in proinflammatory mediators such as TNF-α, IL-1β, IL-6, and IFN-γ, and activation of astrocyte NF-κB. GCM treated-cortical neuronal culture induced a concentration-dependent neuronal death. Pretreatment with α-Klotho decreased TNF-α and IL-6 production, reverted NF-κB activation, and decreased neuronal death induced by GCM. In addition, KL incubation together with GCM + LPS completely reverts the neuronal toxicity induced by low concentration of GCM-LPS. These data suggest an anti-inflammatory and neuroprotective effect of α-Klotho protein in the CNS. This work demonstrated the therapeutic potential of α-Klotho in pathological processes which involves a neuroinflammatory component.
© 2022. The Author(s).
Conflict of interest statement
The authors report no biomedical financial interests or potential conflicts of interest in accordance with the BMC journal policies on author responsibilities of interest policy. We declare that the authors have no competing interests as defined by BMC, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Figures
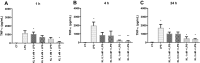
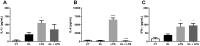
Similar articles
-
Glial-neuronal signaling mechanisms underlying the neuroinflammatory effects of manganese.J Neuroinflammation. 2018 Nov 21;15(1):324. doi: 10.1186/s12974-018-1349-4. J Neuroinflammation. 2018. PMID: 30463564 Free PMC article.
-
Tanshinone IIA suppresses lipopolysaccharide-induced neuroinflammatory responses through NF-κB/MAPKs signaling pathways in human U87 astrocytoma cells.Brain Res Bull. 2020 Nov;164:136-145. doi: 10.1016/j.brainresbull.2020.08.019. Epub 2020 Aug 26. Brain Res Bull. 2020. PMID: 32860868
-
DXXK exerts anti-inflammatory effects by inhibiting the lipopolysaccharide-induced NF-κB/COX-2 signalling pathway and the expression of inflammatory mediators.J Ethnopharmacol. 2016 Feb 3;178:199-208. doi: 10.1016/j.jep.2015.11.016. Epub 2015 Nov 10. J Ethnopharmacol. 2016. PMID: 26571085
-
Anti-Inflammatory Role of the Klotho Protein and Relevance to Aging.Cells. 2024 Aug 24;13(17):1413. doi: 10.3390/cells13171413. Cells. 2024. PMID: 39272986 Free PMC article. Review.
-
Exploiting the neuroprotective effects of α-klotho to tackle ageing- and neurodegeneration-related cognitive dysfunction.Neuronal Signal. 2021 Jun 14;5(2):NS20200101. doi: 10.1042/NS20200101. eCollection 2021 Jun. Neuronal Signal. 2021. PMID: 34194816 Free PMC article. Review.
Cited by
-
Recombinant soluble form of receptor for advanced glycation end products ameliorates microcirculation impairment and neuroinflammation after subarachnoid hemorrhage.Neurotherapeutics. 2024 Mar;21(2):e00312. doi: 10.1016/j.neurot.2023.e00312. Epub 2024 Jan 4. Neurotherapeutics. 2024. PMID: 38177024 Free PMC article.
-
Klotho increases antioxidant defenses in astrocytes and ubiquitin-proteasome activity in neurons.Sci Rep. 2023 Sep 12;13(1):15080. doi: 10.1038/s41598-023-41166-6. Sci Rep. 2023. PMID: 37699938 Free PMC article.
-
Association of serum klotho with cognitive function among individuals with nonalcoholic fatty liver disease.Front Aging Neurosci. 2024 Nov 5;16:1487182. doi: 10.3389/fnagi.2024.1487182. eCollection 2024. Front Aging Neurosci. 2024. PMID: 39563739 Free PMC article.
-
Klotho in age-related cardiovascular diseases: Insights into mitochondrial dysfunction and cell death.Int J Cardiol Heart Vasc. 2025 Mar 8;57:101629. doi: 10.1016/j.ijcha.2025.101629. eCollection 2025 Apr. Int J Cardiol Heart Vasc. 2025. PMID: 40129656 Free PMC article. Review.
-
Association between serum soluble α-klotho and bone mineral density (BMD) in middle-aged and older adults in the United States: a population-based cross-sectional study.Aging Clin Exp Res. 2023 Oct;35(10):2039-2049. doi: 10.1007/s40520-023-02483-y. Epub 2023 Jun 27. Aging Clin Exp Res. 2023. PMID: 37368163
References
-
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. - PubMed
-
- Ohyama Y, Kurabayashi M, Masuda H, Nakamura T, Aihara Y, Kaname T, Suga T, Arai M, Aizawa H, Matsumura Y, et al. Molecular cloning of rat klotho cDNA: Markedly decreased expression of klotho by acute inflammatory stress. Biochem. Biophys. Res. Commun. 1998;251:920–925. - PubMed
-
- Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y. Secreted Klotho protein in sera and CSF: Implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565:143–147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 4050892018-0/Conselho Nacional de Desenvolvimento Científico e Tecnológico
- 2013/10787-8/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 2016/07427-8/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 2018/14289-6/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 2016/22996-9/Fundação de Amparo à Pesquisa do Estado de São Paulo
LinkOut - more resources
Full Text Sources

