Photosynthetic modulation during the diurnal cycle in a unicellular diazotrophic cyanobacterium grown under nitrogen-replete and nitrogen-fixing conditions
- PMID: 36344535
- PMCID: PMC9640542
- DOI: 10.1038/s41598-022-21829-6
Photosynthetic modulation during the diurnal cycle in a unicellular diazotrophic cyanobacterium grown under nitrogen-replete and nitrogen-fixing conditions
Abstract
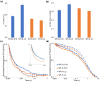
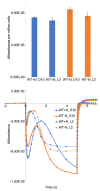
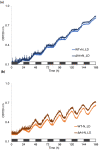
Cyanobacteria are the only oxygenic photosynthetic organisms that can fix nitrogen. In diazotrophic cyanobacteria, the regulation of photosynthesis during the diurnal cycle is hypothesized to be linked with nitrogen fixation and involve the D1 protein isoform PsbA4. The amount of bioavailable nitrogen has a major impact on productivity in aqueous environments. In contrast to low- or nitrogen-fixing (-N) conditions, little data on photosynthetic regulation under nitrogen-replete (+ N) conditions are available. We compared the regulation of photosynthesis under -N and + N conditions during the diurnal cycle in wild type and a psbA4 deletion strain of the unicellular diazotrophic cyanobacterium Cyanothece sp. ATCC 51142. We observed common changes to light harvesting and photosynthetic electron transport during the dark in + N and -N conditions and found that these modifications occur in both diazotrophic and non-diazotrophic cyanobacteria. Nitrogen availability increased PSII titer when cells transitioned from dark to light and promoted growth. Under -N conditions, deletion of PsbA4 modified charge recombination in dark and regulation of PSII titer during dark to light transition. We conclude that darkness impacts the acceptor-side modifications to PSII and photosynthetic electron transport in cyanobacteria independently of the nitrogen-fixing status and the presence of PsbA4.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

