Humoral immunity after second dose of BNT162b2 vaccine in Japanese communities: an observational cross-sectional study, Fukushima Vaccination Community Survey
- PMID: 36344597
- PMCID: PMC9640658
- DOI: 10.1038/s41598-022-21797-x
Humoral immunity after second dose of BNT162b2 vaccine in Japanese communities: an observational cross-sectional study, Fukushima Vaccination Community Survey
Abstract
To reveal waning humoral immunity after second dose BNT162b2 vaccinations in a rural Japanese community and determine factors affecting antibody titers. We aimed to report Immunoglobulin G (IgG) antibody against the SARS-CoV-2 spike (S1) protein levels and neutralizing activity in a large scale community based cohort.
Methods: Participants in the observational cross-sectional study received a second dose of vaccination with BNT162b2 (Pfizer/BioNTech) and were not previously infected with COVID-19. Questionnaire-collected data on sex, age, adverse vaccine reactions, and medical history was obtained.
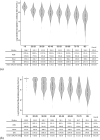
Results: Data from 2496 participants revealed that older age groups reached a low antibody titer 90-120 days after the second vaccination. Neutralizing activity decreased with age; 35 (13.3%) of those aged ≥ 80 years had neutralizing activity under the cut-off value. Neutralizing activity > 179 days from the second vaccination was 11.6% compared to that at < 60 days from the second vaccination. Significantly lower IgG antibody titers and neutralizing activity were associated with age, male sex, increased time from second vaccination, smoking, steroids, immunosuppression, and comorbidities.
Conclusions: Antibody titer decreased substantially over time. Susceptible populations, older people, men, smokers, steroid users, immunosuppression users, and people with three or more comorbidities may require a special protection strategy.
© 2022. The Author(s).
Conflict of interest statement
Kaneko is employed by Medical & Biological Laboratories, Co., (MBL, Tokyo, Japan). MBL imported the testing material used in this research. Kaneko participated in the testing process; however, he did not engage in the research design and analysis. Kobashi and Tsubokura received a research grant from Pfizer Health Research Foundation for research not associated with this work. The remaining authors declare no potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

