A bioinformatics approach to the identification of novel deleterious mutations of human TPMT through validated screening and molecular dynamics
- PMID: 36344599
- PMCID: PMC9640560
- DOI: 10.1038/s41598-022-23488-z
A bioinformatics approach to the identification of novel deleterious mutations of human TPMT through validated screening and molecular dynamics
Abstract
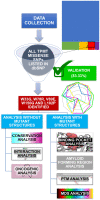
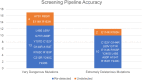
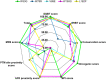
Polymorphisms of Thiopurine S-methyltransferase (TPMT) are known to be associated with leukemia, inflammatory bowel diseases, and more. The objective of the present study was to identify novel deleterious missense SNPs of TPMT through a comprehensive in silico protocol. The initial SNP screening protocol used to identify deleterious SNPs from the pool of all TPMT SNPs in the dbSNP database yielded an accuracy of 83.33% in identifying extremely dangerous variants. Five novel deleterious missense SNPs (W33G, W78R, V89E, W150G, and L182P) of TPMT were identified through the aforementioned screening protocol. These 5 SNPs were then subjected to conservation analysis, interaction analysis, oncogenic and phenotypic analysis, structural analysis, PTM analysis, and molecular dynamics simulations (MDS) analysis to further assess and analyze their deleterious nature. Oncogenic analysis revealed that all five SNPs are oncogenic. MDS analysis revealed that all SNPs are deleterious due to the alterations they cause in the binding energy of the wild-type protein. Plasticity-induced instability caused by most of the mutations as indicated by the MDS results has been hypothesized to be the reason for this alteration. While in vivo or in vitro protocols are more conclusive, they are often more challenging and expensive. Hence, future research endeavors targeted at TPMT polymorphisms and/or their consequences in relevant disease progressions or treatments, through in vitro or in vivo means can give a higher priority to these SNPs rather than considering the massive pool of all SNPs of TPMT.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Elion GB, Hitchings GH. The synthesis of 6-thioguanine. J. Am. Chem. Soc. 1955;77(6):1676. doi: 10.1021/ja01611a082. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

