Incidence of antibiotic resistance genotypes of Vibrio species recovered from selected freshwaters in Southwest Nigeria
- PMID: 36344620
- PMCID: PMC9640555
- DOI: 10.1038/s41598-022-23479-0
Incidence of antibiotic resistance genotypes of Vibrio species recovered from selected freshwaters in Southwest Nigeria
Abstract
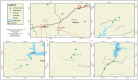
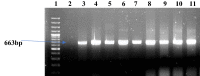
Vibrio species are classified as potent hazards because of their tendency to effect serious diseases like cholera and other gastrointestinal ailments in humans, as well as vibriosis in fish. A total of 144 freshwater samples were aseptically collected monthly across four rivers (Asejire, Ona, Dandaru and Erinle rivers) over a 12-month period from which Vibrio spp. were isolated using culture procedures, confirmed by means of biochemical test as well as Polymerase Chain Reaction (PCR) assay and further characterized for their phenotypic antibiotic susceptibilities and relevant antimicrobial resistant determinants by PCR. Three hundred and fifteen (58%) isolates confirmed across the sampled sites (Asejire = 75, Dandaru = 87, Eleyele = 72, Erinle = 81) showed high resistance against erythromycin-95%, Sulphamethoxazole-94%, rifampicin-92%, doxycycline-82%, tetracycline-75%, amoxicillin-45%, cephalothin-43% and varied susceptibilities to other antibiotics. The multiple antibiotic resistance indices of 97% of the Vibrio isolates were above the 0.2 threshold limit with MAR phenotype pattern E-SUL-RF-TET-DOX (0.38) found to be the most prevalent pattern among the isolates. The distributions of resistance determinant of the tested antibiotics were revealed as follows: sulII 33%, sulI 19% (sulfonamides); blaOXA 27%, ampC 39%, blapse 11% (beta-lactams); tetA 28%, tetE 20%, tet39 8%, (tetracyclines) and strA 39%. aacC2 24%, aphA1 14% (aminoglycosides). Strong positive associations were observed among tetA, sulI, tetE and sulII. This study raises concerns as these selected rivers may contribute to the environmental spread of waterborne diseases and antibiotic resistance genes. Therefore, we recommend environmental context-tailored strategies for monitoring and surveillance of resistance genes so as to safeguard the environment from becoming reservoirs of virulent and infectious Vibrio species.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Parte AC. LPSN—List of prokaryotic names with standing in nomenclature (Bacterio.net), 20 years on. Int. J. Syst. Evol. Microbiol. 2018;68(6):1825–1829. doi: 10.1099/ijsem.0.002786. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

