A novel likely pathogenic variant in the FBXO32 gene associated with dilated cardiomyopathy according to whole‑exome sequencing
- PMID: 36344977
- PMCID: PMC9641816
- DOI: 10.1186/s12920-022-01388-5
A novel likely pathogenic variant in the FBXO32 gene associated with dilated cardiomyopathy according to whole‑exome sequencing
Abstract
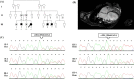
Background: Familial dilated cardiomyopathy (DCM) is a genetic heart disorder characterized by progressive heart failure and sudden cardiac death. Over 250 genes have been reported in association with DCM; nonetheless, the genetic cause of most DCM patients has been unknown. The goal of the present study was to determine the genetic etiology of familial DCM in an Iranian family.
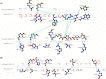
Methods: Whole-exome sequencing was performed to identify the underlying variants in an Iranian consanguineous family with DCM. The presence of the candidate variant was confirmed and screened in available relatives by PCR and Sanger sequencing. The pathogenic effect of the candidate variant was assessed by bioinformatics analysis, homology modeling, and docking.
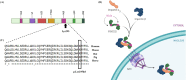
Results: One novel likely pathogenic deletion, c.884_886del: p.Lys295del, in F-box only protein 32 (muscle-specific ubiquitin-E3 ligase protein; FBXO32) was identified. Based on bioinformatics and modeling analysis, c.884_886del was the most probable cause of DCM in the studied family.
Conclusions: Our findings indicate that variants in FBXO32 play a role in recessive DCM. Variants in FBXO32 may disturb the degradation of target proteins in the ubiquitin-proteasome system and lead to severe DCM. We suggest considering this gene variants in patients with recessively inherited DCM.
Keywords: Dilated cardiomyopathy; FBXO32; Genetic; Variant; Whole-exome sequencing.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
A substitution mutation in cardiac ubiquitin ligase, FBXO32, is associated with an autosomal recessive form of dilated cardiomyopathy.BMC Med Genet. 2016 Jan 14;17:3. doi: 10.1186/s12881-016-0267-5. BMC Med Genet. 2016. PMID: 26768247 Free PMC article.
-
Novel pathogenic variant in MED12 causing non-syndromic dilated cardiomyopathy.BMC Med Genomics. 2023 Dec 21;16(1):334. doi: 10.1186/s12920-023-01780-9. BMC Med Genomics. 2023. PMID: 38129817 Free PMC article.
-
FBXO32, encoding a member of the SCF complex, is mutated in dilated cardiomyopathy.Genome Biol. 2016 Jan 11;17:2. doi: 10.1186/s13059-015-0861-4. Genome Biol. 2016. PMID: 26753747 Free PMC article.
-
[A case of dilated cardiomyopathy caused by FHL2 gene variant and a literature review].Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2023 Mar 10;40(3):337-343. doi: 10.3760/cma.j.cn511374-20211230-01025. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2023. PMID: 36854411 Review. Chinese.
-
Genetics of Dilated Cardiomyopathy.Curr Cardiol Rep. 2018 Sep 27;20(11):121. doi: 10.1007/s11886-018-1061-0. Curr Cardiol Rep. 2018. PMID: 30259183 Review.
Cited by
-
Exploring novel natural compound-based therapies for Duchenne muscular dystrophy management: insights from network pharmacology, QSAR modeling, molecular dynamics, and free energy calculations.Front Pharmacol. 2024 Oct 2;15:1395014. doi: 10.3389/fphar.2024.1395014. eCollection 2024. Front Pharmacol. 2024. PMID: 39415830 Free PMC article.
-
Exploring the complex spectrum of dominance and recessiveness in genetic cardiomyopathies.Nat Cardiovasc Res. 2023;2(11):1078-1094. doi: 10.1038/s44161-023-00346-3. Epub 2023 Oct 9. Nat Cardiovasc Res. 2023. PMID: 38666070 Free PMC article.
-
Whole exome sequence reveals genetic profiles of primary cardiomyopathy and genotype-phenotype association in Chinese population.BMC Genomics. 2025 Feb 17;26(1):150. doi: 10.1186/s12864-025-11323-4. BMC Genomics. 2025. PMID: 39962380 Free PMC article.
-
Investigating possible dilated cardiomyopathy targets via bioinformatic analysis.Am J Transl Res. 2023 Jul 15;15(7):4788-4795. eCollection 2023. Am J Transl Res. 2023. PMID: 37560246 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

