A universal predictive and mechanistic urinary peptide signature in acute kidney injury
- PMID: 36345008
- PMCID: PMC9640896
- DOI: 10.1186/s13054-022-04193-9
A universal predictive and mechanistic urinary peptide signature in acute kidney injury
Erratum in
-
Publisher Correction to: A universal predictive and mechanistic urinary peptide signature in acute kidney injury.Crit Care. 2022 Dec 29;26(1):406. doi: 10.1186/s13054-022-04278-5. Crit Care. 2022. PMID: 36581955 Free PMC article. No abstract available.
Abstract
Background: The delayed diagnosis of acute kidney injury (AKI) episodes and the lack of specificity of current single AKI biomarkers hamper its management. Urinary peptidome analysis may help to identify early molecular changes in AKI and grasp its complexity to identify potential targetable molecular pathways.
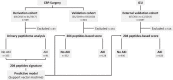
Methods: In derivation and validation cohorts totalizing 1170 major cardiac bypass surgery patients and in an external cohort of 1569 intensive care unit (ICU) patients, a peptide-based score predictive of AKI (7-day KDIGO classification) was developed, validated, and compared to the reference biomarker urinary NGAL and NephroCheck and clinical scores.
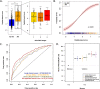
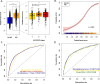
Results: A set of 204 urinary peptides derived from 48 proteins related to hemolysis, inflammation, immune cells trafficking, innate immunity, and cell growth and survival was identified and validated for the early discrimination (< 4 h) of patients according to their risk to develop AKI (OR 6.13 [3.96-9.59], p < 0.001) outperforming reference biomarkers (urinary NGAL and [IGFBP7].[TIMP2] product) and clinical scores. In an external cohort of 1569 ICU patients, performances of the signature were similar (OR 5.92 [4.73-7.45], p < 0.001), and it was also associated with the in-hospital mortality (OR 2.62 [2.05-3.38], p < 0.001).
Conclusions: An overarching AKI physiopathology-driven urinary peptide signature shows significant promise for identifying, at an early stage, patients who will progress to AKI and thus to develop tailored treatments for this frequent and life-threatening condition. Performance of the urine peptide signature is as high as or higher than that of single biomarkers but adds mechanistic information that may help to discriminate sub-phenotypes of AKI offering new therapeutic avenues.
Keywords: Acute kidney injury; Cardiac surgery; Intensive care unit; Prediction; Urinary peptidomics.
© 2022. The Author(s).
Conflict of interest statement
Justyna Siwy and Jochen Metzger are the employee and former employee at Mosaiques diagnostics GmbH (Hannover, Germany), respectively. Harald Mischak is the CEO of Mosaiques diagnostics GmbH. The other authors declare no competing interests.
Figures


References
-
- Mehta RL, Cerdá J, Burdmann EA, Tonelli M, García-García G, Jha V, et al. International society of nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. The Lancet. 2015;385(9987):2616–2643. - PubMed
-
- Vanholder R, Rondeau E, Anders HJ, Carlson N, Fliser D, Kanbay M, et al. EDTAKI: a nephrology and public policy committee platform call for more European involvement in acute kidney injury. Nephrol Dial Transplant. 2022;37(4):740–748. - PubMed
-
- Teo SH, Endre ZH. Biomarkers in acute kidney injury (AKI ) Best Pract Res Clin Anaesthesiol. 2017;31(3):331–344. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

