Deciphering lipid dysregulation in ALS: from mechanisms to translational medicine
- PMID: 36345044
- PMCID: PMC9641964
- DOI: 10.1186/s40035-022-00322-0
Deciphering lipid dysregulation in ALS: from mechanisms to translational medicine
Abstract
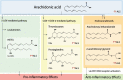
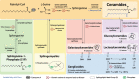
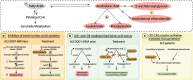
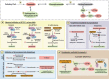
Lipids, defined by low solubility in water and high solubility in nonpolar solvents, can be classified into fatty acids, glycerolipids, glycerophospholipids, sphingolipids, and sterols. Lipids not only regulate integrity and fluidity of biological membranes, but also serve as energy storage and bioactive molecules for signaling. Causal mutations in SPTLC1 (serine palmitoyltransferase long chain subunit 1) gene within the lipogenic pathway have been identified in amyotrophic lateral sclerosis (ALS), a paralytic and fatal motor neuron disease. Furthermore, lipid dysmetabolism within the central nervous system and circulation is associated with ALS. Here, we aim to delineate the diverse roles of different lipid classes and understand how lipid dysmetabolism may contribute to ALS pathogenesis. Among the different lipids, accumulation of ceramides, arachidonic acid, and lysophosphatidylcholine is commonly emerging as detrimental to motor neurons. We end with exploring the potential ALS therapeutics by reducing these toxic lipids.
Keywords: Amyotrophic lateral sclerosis; Arachidonic acid; Ceramides; Cholesterol esters; Eicosanoids; Fatty acids; Lysophosphatidylcholine; Phospholipids; Sphingolipids; Triglycerides.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

