Outlook of various diagnostics and nanodiagnostic techniques for COVID-19
- PMID: 36345412
- PMCID: PMC9632232
- DOI: 10.1016/j.biosx.2022.100276
Outlook of various diagnostics and nanodiagnostic techniques for COVID-19
Abstract
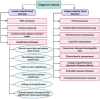
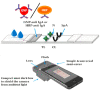
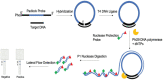
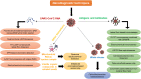
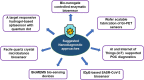
The sudden outbreak of the coronavirus disease 2019 (COVID-19) pandemic has brought to the fore the existing threat of disease-causing pathogens that affect public health all over the world. It has left the best healthcare systems struggling to contain the spread of disease and its consequences. Under challenging circumstances, several innovative technologies have emerged that facilitated quicker diagnosis and treatment. Nanodiagnostic devices are biosensing platforms developed using nanomaterials such as nanoparticles, nanotubes, nanowires, etc. These devices have the edge over conventional techniques such as reverse transcription-polymerase chain reaction (RT-PCR) because of their ease of use, quicker analysis, possible miniaturization, and scope for use in point-of-care (POC) treatment. This review discusses the techniques currently used for COVID-19 diagnosis, emphasizing nanotechnology-based diagnostic devices. The commercialized nanodiagnostic devices in various research and development stages are also reviewed. The advantages of nanodiagnostic devices over other techniques are discussed, along with their limitations. Additionally, the important implications of the utility of nanodiagnostic devices in COVID-19, their prospects for future development for use in clinical and POC settings, and personalized healthcare are also discussed.
Keywords: COVID-19 pandemic; Nanobiosensors; Nanodiagnostics; Nanoparticles; POC devices; SARS-CoV-2.
© 2022 The Author(s).
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Vivek Borse reports financial support was provided by 10.13039/501100006143Department of Science and Technology, Ministry of Science and Technology, Government of India.
Figures



References
-
- #IndiaFightsCorona COVID-19 in India, Vaccination, dashboard, Corona virus tracker | mygov.in [WWW document], n.d. URL https://www.mygov.in/covid-19 (accessed 10.14.2022).
-
- Abbott’s USD 5 15–Minute BinaxNOW COVID-19 Ag card Becomes first diagnostic test with read-result test card to receive FDA EUA - COVID-19-labmedica.com [WWW document] https://www.labmedica.com/covid-19/articles/294784209/abbotts-usd-5-15-m... n.d. URL.
-
- Abdollahi I., Nabahati M., Javanian M., Shirafkan H., Mehraeen R. Can initial chest CT scan predict status and clinical outcomes of COVID-19 infection? A retrospective cohort study. Egypt. J. Radiol. Nucl. Med. 2021;52:1–10. doi: 10.1186/s43055-021-00538-6. - DOI
-
- ACON, n.d. SARS-CoV-2 IgG/IgM Rapid Test Package Insert, Fda.
-
- ADEXUSDx COVID-19 Test Instructions for use [WWW document] https://www.fda.gov/media/149441/download n.d.. Fda. URL.
LinkOut - more resources
Full Text Sources
Miscellaneous
