New insights into anatomical connectivity along the anterior-posterior axis of the human hippocampus using in vivo quantitative fibre tracking
- PMID: 36345716
- PMCID: PMC9643002
- DOI: 10.7554/eLife.76143
New insights into anatomical connectivity along the anterior-posterior axis of the human hippocampus using in vivo quantitative fibre tracking
Abstract
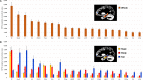
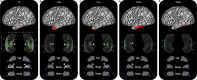
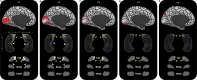
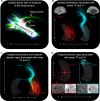
The hippocampus supports multiple cognitive functions including episodic memory. Recent work has highlighted functional differences along the anterior-posterior axis of the human hippocampus, but the neuroanatomical underpinnings of these differences remain unclear. We leveraged track-density imaging to systematically examine anatomical connectivity between the cortical mantle and the anterior-posterior axis of the in vivo human hippocampus. We first identified the most highly connected cortical areas and detailed the degree to which they preferentially connect along the anterior-posterior axis of the hippocampus. Then, using a tractography pipeline specifically tailored to measure the location and density of streamline endpoints within the hippocampus, we characterised where these cortical areas preferentially connect within the hippocampus. Our results provide new and detailed insights into how specific regions along the anterior-posterior axis of the hippocampus are associated with different cortical inputs/outputs and provide evidence that both gradients and circumscribed areas of dense extrinsic anatomical connectivity exist within the human hippocampus. These findings inform conceptual debates in the field and emphasise the importance of considering the hippocampus as a heterogeneous structure. Overall, our results represent a major advance in our ability to map the anatomical connectivity of the human hippocampus in vivo and inform our understanding of the neural architecture of hippocampal-dependent memory systems in the human brain.
Keywords: diffusion-weighted imaging; hippocampal subfields; hippocampus; human; neuroscience; structural connectivity; track-weighted imaging.
Plain language summary
The brain allows us to perceive and interact with our environment and to create and recall memories about our day-to-day lives. A sea-horse shaped structure in the brain, called the hippocampus, is critical for translating our perceptions into memories, and it does so in coordination with other brain regions. For example, different regions of the cerebral cortex (the outer layer of the brain) support different aspects of cognition, and pathways of information flow between the cerebral cortex and hippocampus underpin the healthy functioning of memory. Decades of research conducted into the brains of non-human primates show that specific regions of the cerebral cortex anatomically connect with different parts of the hippocampus to support this information flow. These insights form the foundation for existing theoretical models of how networks of neurons in the hippocampus and the cerebral cortex are connected. However, the human cerebral cortex has greatly expanded during our evolution, meaning that patterns of connectivity in the human brain may diverge from those in the brains of non-human primates. Deciphering human brain circuits in greater detail is crucial if we are to gain a better understanding of the structure and operation of the healthy human brain. However, obtaining comprehensive maps of anatomical connections between the hippocampus and cerebral cortex has been hampered by technical limitations. For example, magnetic resonance imaging (MRI), an approach that can be used to study the living human brain, suffers from insufficient image resolution. To overcome these issues, Dalton et al. used an imaging technique called diffusion weighted imaging which is used to study white matter pathways in the brain. They developed a tailored approach to create high-resolution maps showing how the hippocampus anatomically connects with the cerebral cortex in the healthy human brain. Dalton et al. produced detailed maps illustrating which areas of the cerebral cortex have high anatomical connectivity with the hippocampus and how different parts of the hippocampus preferentially connect to different neural circuits in the cortex. For example, the experiments demonstrate that highly connected areas in a cortical region called the temporal cortex connect to very specific, circumscribed regions within the hippocampus. These findings suggest that the hippocampus may consist of different neural circuits, each preferentially linked to defined areas of the cortex which are, in turn, associated with specific aspects of cognition. These observations further our knowledge of hippocampal-dependant memory circuits in the human brain and provide a foundation for the study of memory decline in aging and neurodegenerative diseases.
© 2022, Dalton et al.
Conflict of interest statement
MD, AD, JL No competing interests declared, FC is listed as one of the inventors in a patent awarded for the track-density imaging method
Figures









Comment in
- doi: 10.7554/eLife.83718
Similar articles
-
Mapping brain-wide excitatory projectome of primate prefrontal cortex at submicron resolution and comparison with diffusion tractography.Elife. 2022 May 20;11:e72534. doi: 10.7554/eLife.72534. Elife. 2022. PMID: 35593765 Free PMC article.
-
Sleep Spindles Promote the Restructuring of Memory Representations in Ventromedial Prefrontal Cortex through Enhanced Hippocampal-Cortical Functional Connectivity.J Neurosci. 2020 Feb 26;40(9):1909-1919. doi: 10.1523/JNEUROSCI.1946-19.2020. Epub 2020 Jan 20. J Neurosci. 2020. PMID: 31959699 Free PMC article.
-
Longitudinal hippocampal axis in large-scale cortical systems underlying development and episodic memory.Proc Natl Acad Sci U S A. 2024 Oct 29;121(44):e2403015121. doi: 10.1073/pnas.2403015121. Epub 2024 Oct 22. Proc Natl Acad Sci U S A. 2024. PMID: 39436664 Free PMC article.
-
Meta-analytic and functional connectivity evidence from functional magnetic resonance imaging for an anterior to posterior gradient of function along the hippocampal axis.Hippocampus. 2020 May;30(5):456-471. doi: 10.1002/hipo.23164. Epub 2019 Oct 7. Hippocampus. 2020. PMID: 31589003 Review.
-
Functional MRI evidence for distinctive binding and consolidation pathways for face-name associations: analysis of activation maps and BOLD response amplitudes.Top Magn Reson Imaging. 2009 Oct;20(5):271-8. doi: 10.1097/RMR.0b013e3181e8f1f9. Top Magn Reson Imaging. 2009. PMID: 20859188 Free PMC article. Review.
Cited by
-
Posterior hippocampal CA2/3 volume is associated with autobiographical memory recall ability in lower performing individuals.Sci Rep. 2023 May 16;13(1):7924. doi: 10.1038/s41598-023-35127-2. Sci Rep. 2023. PMID: 37193748 Free PMC article.
-
Structural equation modeling of treatment-related changes in neural connectivity for youth with PTSD.J Affect Disord. 2023 Aug 1;334:50-59. doi: 10.1016/j.jad.2023.04.066. Epub 2023 Apr 30. J Affect Disord. 2023. PMID: 37127117 Free PMC article.
-
Multi-modal MRI of hippocampal morphometry and connectivity after pediatric severe TBI.Brain Imaging Behav. 2024 Feb;18(1):159-170. doi: 10.1007/s11682-023-00818-x. Epub 2023 Nov 13. Brain Imaging Behav. 2024. PMID: 37955810 Free PMC article.
-
Tau follows principal axes of functional and structural brain organization in Alzheimer's disease.Nat Commun. 2024 Jun 12;15(1):5031. doi: 10.1038/s41467-024-49300-2. Nat Commun. 2024. PMID: 38866759 Free PMC article.
-
Neural cell diversity in the light of single-cell transcriptomics.Transcription. 2023 Jun-Oct;14(3-5):158-176. doi: 10.1080/21541264.2023.2295044. Epub 2024 Jan 23. Transcription. 2023. PMID: 38229529 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

