AMX - the highly automated macromolecular crystallography (17-ID-1) beamline at the NSLS-II
- PMID: 36345756
- PMCID: PMC9641562
- DOI: 10.1107/S1600577522009377
AMX - the highly automated macromolecular crystallography (17-ID-1) beamline at the NSLS-II
Abstract
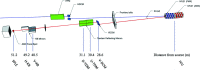
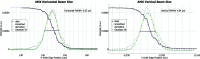
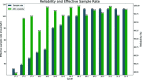
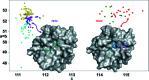
The highly automated macromolecular crystallography beamline AMX/17-ID-1 is an undulator-based high-intensity (>5 × 1012 photons s-1), micro-focus (7 µm × 5 µm), low-divergence (1 mrad × 0.35 mrad) energy-tunable (5-18 keV) beamline at the NSLS-II, Brookhaven National Laboratory, Upton, NY, USA. It is one of the three life science beamlines constructed by the NIH under the ABBIX project and it shares sector 17-ID with the FMX beamline, the frontier micro-focus macromolecular crystallography beamline. AMX saw first light in March 2016 and started general user operation in February 2017. At AMX, emphasis has been placed on high throughput, high capacity, and automation to enable data collection from the most challenging projects using an intense micro-focus beam. Here, the current state and capabilities of the beamline are reported, and the different macromolecular crystallography experiments that are routinely performed at AMX/17-ID-1 as well as some plans for the near future are presented.
Keywords: automation; beamline; high throughput; macromolecular crystallography; micro-beam; real-time feedback; synchrotron source.
open access.
Figures





References
-
- Allan, D., Caswell, T., Campbell, S. & Rakitin, M. (2019). Synchrotron Radiat. News, 32(3), 19–22.
-
- Arndt, U. W. & Wonacott, A. J. (1977). Rotation Method in Crystallography. Amsterdam: North-Holland.
-
- Bowler, M. W., Nurizzo, D., Barrett, R., Beteva, A., Bodin, M., Caserotto, H., Delagenière, S., Dobias, F., Flot, D., Giraud, T., Guichard, N., Guijarro, M., Lentini, M., Leonard, G. A., McSweeney, S., Oskarsson, M., Schmidt, W., Snigirev, A., von Stetten, D., Surr, J., Svensson, O., Theveneau, P. & Mueller-Dieckmann, C. (2015). J. Synchrotron Rad. 22, 1540–1547. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

