The effect of induction immunosuppression for kidney transplant on the latent HIV reservoir
- PMID: 36345940
- PMCID: PMC9675561
- DOI: 10.1172/jci.insight.162968
The effect of induction immunosuppression for kidney transplant on the latent HIV reservoir
Abstract
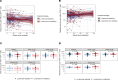
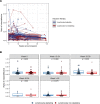
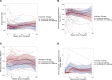
The HIV latent viral reservoir (LVR) remains a major challenge in the effort to find a cure for HIV. There is interest in lymphocyte-depleting agents, used in solid organ and bone marrow transplantation to reduce the LVR. This study evaluated the LVR and T cell receptor repertoire in HIV-infected kidney transplant recipients using intact proviral DNA assay and T cell receptor sequencing in patients receiving lymphocyte-depleting or lymphocyte-nondepleting immunosuppression induction therapy. CD4+ T cells and intact and defective provirus frequencies decreased following lymphocyte-depleting induction therapy but rebounded to near baseline levels within 1 year after induction. In contrast, these biomarkers were relatively stable over time in the lymphocyte-nondepleting group. The lymphocyte-depleting group had early TCRβ repertoire turnover and newly detected and expanded clones compared with the lymphocyte-nondepleting group. No differences were observed in TCRβ clonality and repertoire richness between groups. These findings suggest that, even with significant decreases in the overall size of the circulating LVR, the reservoir can be reconstituted in a relatively short period of time. These results, while from a relatively unique population, suggest that curative strategies aimed at depleting the HIV LVR will need to achieve specific and durable levels of HIV-infected T cell depletion.
Keywords: AIDS/HIV; Organ transplantation; T cells.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

