Anti-pan-neurofascin antibodies induce subclass-related complement activation and nodo-paranodal damage
- PMID: 36346134
- PMCID: PMC10151189
- DOI: 10.1093/brain/awac418
Anti-pan-neurofascin antibodies induce subclass-related complement activation and nodo-paranodal damage
Abstract
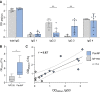
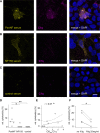
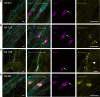
Autoimmune neuropathy associated with antibodies against pan-neurofascin is a new subtype of nodo-paranodopathy. It is relevant because it is associated with high morbidity and mortality. Affected patients often require intensive care unit treatment for several months, and data on the reversibility and long-term prognosis are limited. The pathogenicity including IgG subclass-associated mechanisms has not been unravelled, nor directly compared to anti-neurofascin-155 IgG4-related pathology. Understanding the underlying pathology might have a direct impact on treatment of these severely affected patients. By a multicentre combined prospective and retrospective approach, we provide clinical data of a large cohort of patients with anti-neurofascin-associated neuropathy (n = 18) including longitudinal titre and neurofilament light chain assessment via Ella® and relate clinical data to in vitro pathogenicity studies of anti-neurofascin antibodies. We assessed antibody binding characteristics and the pathogenic effects of anti-pan-neurofascin versus neurofascin-155 antibodies on living myelinating dorsal root ganglia co-cultures. Additionally, we analysed the IgG subclass profile and the complement binding capacity and effector functions considering the effects of intravenous immunoglobulin preparations via enzyme-linked immunosorbent and cell-based assays. In contrast to chronic neurofascin-155 IgG4-associated neuropathy, anti-pan-neurofascin-associated disease presented with a high morbidity and mortality, but as a monophasic and potentially reversible disorder. During follow-up, antibodies were no longer detectable in 8 of 11 patients. Anti-pan-neurofascin had direct access to the nodes of Ranvier in myelinating cultures titre-dependently, most probably inducing this severe phenotype. Antibody preincubation led to impaired paranode formation, destruction of paranodal architecture and alterations on paranodal myelin and sensory neurons in the cultures, with more severe effects than neurofascin-155 antibodies. Besides IgG4, subclass IgG3 was detected and associated with complement binding and cytotoxic effects in vitro. As a possible correlate of axonal damage in vivo, we detected highly increased serum neurofilament light chain levels (sNF-L), correlating to serum C3a. Still, sNF-L was not identified as a marker for poor prognosis, but rather as an intra- and interindividual marker for acuteness, severity and course, with a strong decrease during recovery. Our data provide evidence that anti-pan-neurofascin antibodies directly attack the node and induce severe and acute, but potentially reversible, nodo-paranodal pathology, possibly involving complement-mediated mechanisms. Screening for autoantibodies thus is crucial to identify this subset of patients who benefit from early antibody-depleting therapy. Titre and sNF-L might serve as valuable follow-up parameters. The prospect of a favourable outcome has high relevance for physicians, patients and relatives during months of critical care.
Keywords: autoantibodies; complement; neurofascin; neurofilament light chain; nodo-paranodopathy.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
L.A., F.L., C.S. and K.D. work for an academic institution offering commercial antibody diagnostics. I.A. has received travel grants from Biogen Idec and Guthy-Jackson Charitable Foundation, served on scientific advisory boards for Roche, Alexion, Merck and received research support from Diamed, none related to this manuscript. A.L.F. received research funding from Georgius Agricola Stiftung Ruhr, Ruhr University Bochum (FoRUM-program) and GBS CIDP Foundation International. She owns shares of Fresenius SE & Co., Gilead Sciences, Medtronic PLC and Novartis AG. F.L. serves or has served on advisory boards for Biogen, Roche and Alexion and received speaker honoraria from Roche, Biogen, Grifols, Alexion, Desitin and Novartis. He serves as editorial board member for Neurology N2. J.M. received travel grants from Biogen idec. His research is funded by Klaus Tschira Foundation, Hertie Foundation, Novartis, Biogen and Ruhr-University, Bochum (FoRUM-Program), none related to this work. K.P. received travel grants and speaker honoraria from Biogen Idec and Bayer Schering, Novartis, Celgene and Grifols and has participated in advisory board meeting for Celgene, none related to this manuscript. C.S. has served on scientific advisory boards for Akcea, Algiax, Air Liquide, Bayer, Grifols, Ipsen, LFB, Immunic, Merz, Pfizer, Roche and Takeda. She received speaker honoraria from Akcea, Alnylam Amicus, Grifols, Pfizer and Teva. She serves or has served as a journal editor, associate editor or editorial advisory board member for the
Figures



References
-
- van den Berg B, Walgaard C, Drenthen J, Fokke C, Jacobs BC, van Doorn PA. Guillain–Barré syndrome: Pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol. 2014;10:469–482. - PubMed
-
- Devaux JJ, Odaka M, Yuki N. Nodal proteins are target antigens in Guillain–Barré syndrome. J Peripher Nerv Syst. 2012;17:62–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

