Intestinal Dominance by Multidrug-Resistant Bacteria in Pediatric Liver Transplant Patients
- PMID: 36346231
- PMCID: PMC9769714
- DOI: 10.1128/spectrum.02842-22
Intestinal Dominance by Multidrug-Resistant Bacteria in Pediatric Liver Transplant Patients
Abstract
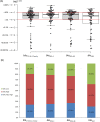
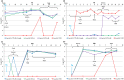
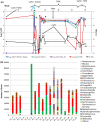
Pediatric liver transplantation (PLTx) is commonly associated with extensive antibiotic treatments that can produce gut microbiome alterations and open the way to dominance by multidrug-resistant organisms (MDROs). In this study, the relationship between intestinal Relative Loads (RLs) of β-lactamase genes, antibiotic consumption, microbiome disruption, and the extraintestinal dissemination of MDROs among PLTx patients is investigated. 28 PLTx patients were included, from whom 169 rectal swabs were collected. Total DNA was extracted and blaCTX-M-1-Family, blaOXA-1, blaOXA-48, and blaVIM were quantified via quantitative polymerase chain reaction (qPCR) and normalized to the total bacterial load (16SrRNA) through LogΔΔCt to determine the RLs. 16SrRNA sequencing was performed for 18 samples, and metagenomic sequencing was performed for 2. Patients' clinical data were retrieved from the hospital's database. At least one of the genes tested were detected in all of the patients. The RLs for blaCTX-M-1-Family, blaOXA-1, blaOXA-48, and blaVIM were higher than 1% of the total bacterial population in 67 (80.73%), 56 (78.87%), 57 (77.03%) and 39 (61.9%) samples, respectively. High RLs for blaCTX-M-1-Family, blaOXA-1, and/or blaOXA-48, were positively associated with the consumption of carbapenems with trimethoprim-sulfamethoxazole and coincided with low diversity in the gut microbiome. Low RLs were associated with the consumption of noncarbapenem β-lactams with aminoglycosides (P < 0.05). Extraintestinal isolates harboring the same gene(s) as those detected intraintestinally were found in 18 samples, and the RLs of the respective swabs were high. We demonstrated a relationship between the consumption of carbapenems with trimethoprim-sulfamethoxazole, intestinal dominance by MDROs and extraintestinal spread of these organisms among PLTx patients. IMPORTANCE In this study, we track the relative intestinal loads of antibiotic resistance genes among pediatric liver transplant patients and determine the relationship between this load, antibiotic consumption, and infections caused by antibiotic-resistant organisms. We demonstrate that the consumption of broad spectrum antibiotics increase this load and decrease the gut microbial diversity among these patients. Moreover, the high loads of resistance genes were related to the extraintestinal spread of multidrug-resistant organisms. Together, our data show that the tracking of the relative intestinal loads of antibiotic resistance genes can be used as a biomarker that has the potential to stop the extraintestinal spread of antibiotic-resistant bacteria via the measurement of the intestinal dominance of these organisms, thereby allowing for the application of preventive measures.
Keywords: antibiotic consumption; extraintestinal multidrug-resistant organisms; intestinal dominance; microbiome; pediatric liver transplant patients; qPCR; relative intestinal load; β-lactamase genes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

