Characterization of MxiE- and H-NS-Dependent Expression of ipaH7.8, ospC1, yccE, and yfdF in Shigella flexneri
- PMID: 36346241
- PMCID: PMC9769918
- DOI: 10.1128/msphere.00485-22
Characterization of MxiE- and H-NS-Dependent Expression of ipaH7.8, ospC1, yccE, and yfdF in Shigella flexneri
Abstract
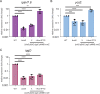
Shigella flexneri uses a type 3 secretion system (T3SS) apparatus to inject virulence effector proteins into the host cell cytosol. Upon host cell contact, MxiE, an S. flexneri AraC-like transcriptional regulator, is required for the expression of a subset of T3SS effector genes encoded on the large virulence plasmid. Here, we defined the MxiE regulon using RNA-seq. We identified virulence plasmid- and chromosome-encoded genes that are activated in response to type 3 secretion in a MxiE-dependent manner. Bioinformatic analysis revealed that similar to previously known MxiE-dependent genes, chromosome-encoded genes yccE and yfdF contain a regulatory element known as the MxiE box, which is required for their MxiE-dependent expression. The significant AT enrichment of MxiE-dependent genes suggested the involvement of H-NS. Using a dominant negative H-NS system, we demonstrate that H-NS silences the expression of MxiE-dependent genes located on the virulence plasmid (ipaH7.8 and ospC1) and the chromosome (yccE and yfdF). Furthermore, we show that MxiE is no longer required for the expression of ipaH7.8, ospC1, yccE, and yfdF when H-NS silencing is relieved. Finally, we show that the H-NS anti-silencer VirB is not required for ipaH7.8 and yccE expression upon MxiE/IpgC overexpression. Based on these genetic studies, we propose a model of MxiE-dependent gene regulation in which MxiE counteracts H-NS-mediated silencing. IMPORTANCE The expression of horizontally acquired genes, including virulence genes, is subject to complex regulation involving xenogeneic silencing proteins, and counter-silencing mechanisms. The pathogenic properties of Shigella flexneri mainly rely on the acquisition of the type 3 secretion system (T3SS) and cognate effector proteins, whose expression is repressed by the xenogeneic silencing protein H-NS. Based on previous studies, releasing H-NS-mediated silencing mainly relies on two mechanisms involving (i) a temperature shift leading to the release of H-NS at the virF promoter, and (ii) the virulence factor VirB, which dislodges H-NS upon binding to specific motifs upstream of virulence genes, including those encoding the T3SS. In this study, we provide genetic evidence supporting the notion that, in addition to VirB, the AraC family member MxiE also contributes to releasing H-NS-mediated silencing in S. flexneri.
Keywords: H-NS; MxiE; Shigella; T3SS; anti-silencing; silencing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


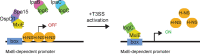
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

