Effect of noradrenaline on propofol-induced mitochondrial dysfunction in human skeletal muscle cells
- PMID: 36346511
- PMCID: PMC9643307
- DOI: 10.1186/s40635-022-00474-3
Effect of noradrenaline on propofol-induced mitochondrial dysfunction in human skeletal muscle cells
Abstract
Background: Mitochondrial dysfunction is a hallmark of both critical illness and propofol infusion syndrome and its severity seems to be proportional to the doses of noradrenaline, which patients are receiving. We comprehensively studied the effects of noradrenaline on cellular bioenergetics and mitochondrial biology in human skeletal muscle cells with and without propofol-induced mitochondrial dysfunction.
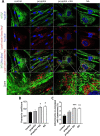
Methods: Human skeletal muscle cells were isolated from vastus lateralis biopsies from patients undergoing elective hip replacement surgery (n = 14) or healthy volunteers (n = 4). After long-term (96 h) exposure to propofol (10 µg/mL), noradrenaline (100 µM), or both, energy metabolism was assessed by extracellular flux analysis and substrate oxidation assays using [14C] palmitic and [14C(U)] lactic acid. Mitochondrial membrane potential, morphology and reactive oxygen species production were analysed by confocal laser scanning microscopy. Mitochondrial mass was assessed both spectrophotometrically and by confocal laser scanning microscopy.
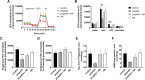
Results: Propofol moderately reduced mitochondrial mass and induced bioenergetic dysfunction, such as a reduction of maximum electron transfer chain capacity, ATP synthesis and profound inhibition of exogenous fatty acid oxidation. Noradrenaline exposure increased mitochondrial network size and turnover in both propofol treated and untreated cells as apparent from increased co-localization with lysosomes. After adjustment to mitochondrial mass, noradrenaline did not affect mitochondrial functional parameters in naïve cells, but it significantly reduced the degree of mitochondrial dysfunction induced by propofol co-exposure. The fatty acid oxidation capacity was restored almost completely by noradrenaline co-exposure, most likely due to restoration of the capacity to transfer long-chain fatty acid to mitochondria. Both propofol and noradrenaline reduced mitochondrial membrane potential and increased reactive oxygen species production, but their effects were not additive.
Conclusions: Noradrenaline prevents rather than aggravates propofol-induced impairment of mitochondrial functions in human skeletal muscle cells. Its effects on bioenergetic dysfunctions of other origins, such as sepsis, remain to be demonstrated.
Keywords: Critical illness; Mitochondrial dysfunction; Noradrenaline; Propofol infusion syndrome; Skeletal muscle.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

