The XINDI Study: A Randomized Phase III Clinical Trial Evaluating the Efficacy and Safety of Safinamide as Add-On Therapy to Levodopa in Chinese Patients with Parkinson's Disease with Motor Fluctuations
- PMID: 36346534
- PMCID: PMC9641300
- DOI: 10.1007/s40263-022-00958-6
The XINDI Study: A Randomized Phase III Clinical Trial Evaluating the Efficacy and Safety of Safinamide as Add-On Therapy to Levodopa in Chinese Patients with Parkinson's Disease with Motor Fluctuations
Abstract
Background: Levodopa remains the gold standard for the treatment of Parkinson's disease, but its long-term use is associated with motor complications whose management is still a significant challenge. Safinamide is a multimodal drug with proven efficacy as an adjunct to levodopa.
Objective: The objective of this study was to investigate the efficacy and safety of safinamide as an add-on to levodopa in Chinese patients with Parkinson's disease with motor fluctuations.
Methods: The XINDI study was a phase III, randomized, double-blind, placebo-controlled, multicenter study, with a 2-week screening period and a 16-week treatment period. The starting dose of safinamide (or placebo) was 50 mg once daily, increased to 100 mg once daily at day 15. Patients aged ≥ 18 years, with idiopathic Parkinson's disease of >3 years duration, Hoehn and Yahr stage 1-4, and daily OFF time ≥ 1.5 h, were eligible. Patients should follow a stable oral levodopa regimen and may receive concomitant treatment with stable doses of other anti-Parkinson drugs, except monoamine oxidase-B inhibitors. Patients with severe disabling peak-dose or biphasic dyskinesia, unpredictable or widely swinging fluctuations, other forms of parkinsonism, a history of dementia or severe cognitive dysfunction, major psychiatric illnesses, and/or clinically significant medical illnesses were excluded. The primary efficacy endpoint was the change from baseline to week 16 in the mean daily OFF time. Secondary efficacy endpoints included the Unified Parkinson's Disease Rating Scale, the Numerical Rating Scale, the Clinical Global Impression scale, and the 39-Item Parkinson's Disease Questionnaire scale. The statistical analysis of the efficacy parameters was conducted using an analysis of co-variance, except for the Clinical Global Impression scale scores that were assessed using the Wilcoxon-Mann-Whitney test. Safety was evaluated through the frequency of adverse events and serious adverse events, physical examination, vital signs, 12-lead electrocardiograms, and laboratory exams. All safety endpoints were summarized using descriptive statistics.
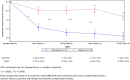
Results: The trial enrolled 307 patients. At week 16, the difference in the change of the mean total daily OFF time between safinamide and placebo groups was 1.10 h (p < 0.0001). This change was significantly greater in the safinamide group starting from week 2, suggesting a rapid onset of drug efficacy. ON time, Unified Parkinson's Disease Rating Scale, Clinical Global Impression scale, and the 39-Item Parkinson's Disease Questionnaire showed statistically significant improvements. There were no significant between-group differences for adverse events or serious adverse events.
Conclusions: Safinamide, as add-on therapy to levodopa, significantly reduced motor fluctuations and improved motor symptoms and quality of life of Chinese patients with idiopathic Parkinson's disease. The improvements observed in the Unified Parkinson's Disease Rating Scale total and motor scores were also clinically significant. No safety concerns were identified, confirming the good tolerability profile of the drug.
Clinical trial registration: NCT03881371, registered on 19 March, 2019, https://clinicaltrials.gov/NCT03881371 .
© 2022. The Author(s).
Conflict of interest statement
The study and its open access publication was funded by Zambon S.p.A. Carlo Cattaneo is an employee at Zambon S.p.A. Qianqian Wei, Yuyan Tan, Pingyi Xu, Enxiang Tao, Zuneng Lu, Xiaoping Pan, Baojun Wang, Chunfeng Liu, Xueshuang Dong, Yuling Tian, and Xin Sun have no conflicts of interest that are directly relevant to the content of this article.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

