Hypoxic storage of murine red blood cells improves energy metabolism and post-transfusion recoveries
- PMID: 36346885
- PMCID: PMC9918384
- DOI: 10.2450/2022.0172-22
Hypoxic storage of murine red blood cells improves energy metabolism and post-transfusion recoveries
Abstract
Background: The Red blood cell (RBC) storage lesion results in decreased circulation and function of transfused RBCs. Elevated oxidant stress and impaired energy metabolism are a hallmark of the storage lesion in both human and murine RBCs. Although human studies don't suffer concerns that findings may not translate, they do suffer from genetic and environmental variability amongst subjects. Murine models can control for genetics, environment, and much interventional experimentation can be carried out in mice that is neither technically feasible nor ethical in humans. However, murine models are only useful to the extent that they have similar biology to humans. Hypoxic storage has been shown to mitigate the storage lesion in human RBCs, but has not been investigated in mice.
Materials and methods: RBCs from a C57BL6/J mouse strain were stored under normoxic (untreated) or hypoxic conditions (SO2 ~ 26%) for 1h, 7 and 12 days. Samples were tested for metabolomics at steady state, tracing experiments with 1,2,3-13C3-glucose, proteomics and end of storage post transfusion recovery.
Results: Hypoxic storage improved post-transfusion recovery and energy metabolism, including increased steady state and 13C3-labeled metabolites from glycolysis, high energy purines (adenosine triphosphate) and 2,3-diphospholgycerate. Hypoxic storage promoted glutaminolysis, increased glutathione pools, and was accompanied by elevation in the levels of free fatty acids and acyl-carnitines.
Discussion: This study isolates hypoxia, as a single independent variable, and shows similar effects as seen in human studies. These findings also demonstrate the translatability of murine models for hypoxic RBC storage and provide a pre-clinical platform for ongoing study.
Conflict of interest statement
The Authors declare that AD is a founder of Omix Technologies Inc. and Altis Biosciences LLC. He is also a consultant for Rubius Inc. Macopharma and Forma Inc. AD is an advisory board member of Hemanext Inc, a company that is developing a product for hypoxic storage of human RBCs. JCZ is a consultant for Rubius Inc. JCZ is a cofounder and the chief scientific officer for Svalinn therapeutics, a company whose focus is unrelated to the current work. All the other Authors disclose no conflicts of interest relevant to this study.
Figures
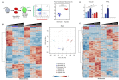


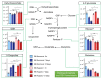

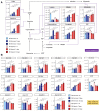
Comment in
-
Benefits of hypoxic storage of red blood cells.Blood Transfus. 2023 Jan;21(1):1-2. doi: 10.2450/2023.0229-22. Blood Transfus. 2023. PMID: 36763908 Free PMC article. No abstract available.
Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Prolonged storage of packed red blood cells for blood transfusion.Cochrane Database Syst Rev. 2015 Jul 14;2015(7):CD009330. doi: 10.1002/14651858.CD009330.pub2. Cochrane Database Syst Rev. 2015. PMID: 26171902 Free PMC article.
-
Restrictive versus liberal red blood cell transfusion strategies for people with haematological malignancies treated with intensive chemotherapy or radiotherapy, or both, with or without haematopoietic stem cell support.Cochrane Database Syst Rev. 2017 Jan 27;1(1):CD011305. doi: 10.1002/14651858.CD011305.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2024 May 23;5:CD011305. doi: 10.1002/14651858.CD011305.pub3. PMID: 28128441 Free PMC article. Updated.
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.Health Technol Assess. 2007 Nov;11(45):iii-iv, ix-221. doi: 10.3310/hta11450. Health Technol Assess. 2007. PMID: 17999840
Cited by
-
Complement C3 and marginal zone B cells promote IgG-mediated enhancement of RBC alloimmunization in mice.J Clin Invest. 2024 Apr 15;134(8):e167665. doi: 10.1172/JCI167665. J Clin Invest. 2024. PMID: 38618959 Free PMC article.
-
Red Blood Cell Metabolism In Vivo and In Vitro.Metabolites. 2023 Jun 27;13(7):793. doi: 10.3390/metabo13070793. Metabolites. 2023. PMID: 37512500 Free PMC article. Review.
-
Molecular modifications to mitigate oxidative stress and improve red blood cell storability.Front Physiol. 2024 Oct 30;15:1499308. doi: 10.3389/fphys.2024.1499308. eCollection 2024. Front Physiol. 2024. PMID: 39539958 Free PMC article. Review.
-
A spleen is required for antibody mediated immune enhancement but not for RBC clearance or antigen-modulation in mice.Transfusion. 2025 Mar;65(3):453-458. doi: 10.1111/trf.18148. Epub 2025 Jan 31. Transfusion. 2025. PMID: 39887361 Free PMC article.
-
Sphingosine 1-phosphate has a negative effect on RBC storage quality.Blood Adv. 2023 Apr 25;7(8):1379-1393. doi: 10.1182/bloodadvances.2022008936. Blood Adv. 2023. PMID: 36469038 Free PMC article.
References
-
- Tzounakas VL, Kriebardis AG, Georgatzakou HT, Foudoulaki-Paparizos LE, Dzieciatkowska M, Wither MJ, et al. Glucose 6-phosphate dehydrogenase deficient subjects may be better “storers” than donors of red blood cells. Free Radic Biol Med. 2016;96:152–165. doi: 10.1016/j.freeradbiomed.2016.04.005. - DOI - PubMed
-
- D’Alessandro A, Fu X, Kanias T, Reisz JA, Culp-Hill R, Guo Y, et al. Donor sex, age and ethnicity impact stored red blood cell antioxidant metabolism through mechanisms in part explained by glucose 6-phosphate dehydrogenase levels and activity. Haematologica. 2021;106:1290–1302. doi: 10.3324/haematol.2020.246603. - DOI - PMC - PubMed
-
- Hazegh K, Fang F, Bravo MD, Tran JQ, Muench MO, Jackman RP, et al. Blood donor obesity is associated with changes in red blood cell metabolism and susceptibility to hemolysis in cold storage and in response to osmotic and oxidative stress. Transfusion. 2021;61:435–448. doi: 10.1111/trf.16168. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
