Reduced mitochondria provide an essential function for the cytosolic methionine cycle
- PMID: 36347252
- PMCID: PMC9746703
- DOI: 10.1016/j.cub.2022.10.028
Reduced mitochondria provide an essential function for the cytosolic methionine cycle
Abstract
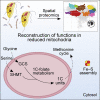
The loss of mitochondria in oxymonad protists has been associated with the redirection of the essential Fe-S cluster assembly to the cytosol. Yet as our knowledge of diverse free-living protists broadens, the list of functions of their mitochondrial-related organelles (MROs) expands. We revealed another such function in the closest oxymonad relative, Paratrimastix pyriformis, after we solved the proteome of its MRO with high accuracy, using localization of organelle proteins by isotope tagging (LOPIT). The newly assigned enzymes connect to the glycine cleavage system (GCS) and produce folate derivatives with one-carbon units and formate. These are likely to be used by the cytosolic methionine cycle involved in S-adenosyl methionine recycling. The data provide consistency with the presence of the GCS in MROs of free-living species and its absence in most endobionts, which typically lose the methionine cycle and, in the case of oxymonads, the mitochondria.
Keywords: LOPIT; Paratrimastix; glycine cleavage system; methionine cycle; mitochondrion-related organelle; one-carbon metabolism; proteome; spatial proteomics.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




Comment in
-
Eukaryotic evolution: Spatial proteomics sheds light on mitochondrial reduction.Curr Biol. 2022 Dec 5;32(23):R1308-R1311. doi: 10.1016/j.cub.2022.10.039. Curr Biol. 2022. PMID: 36473440
References
-
- Karnkowska A., Vacek V., Zubáčová Z., Treitli S.C., Petrželková R., Eme L., Novák L., Žárský V., Barlow L.D., Herman E.K., et al. A eukaryote without a mitochondrial organelle. Curr. Biol. 2016;26:1274–1284. - PubMed
-
- Roger A.J., Muñoz-Gómez S.A., Kamikawa R. The origin and diversification of mitochondria. Curr. Biol. 2017;27:R1177–R1192. - PubMed
-
- Santos H.J., Makiuchi T., Nozaki T. Reinventing an organelle: the reduced mitochondrion in parasitic protists. Trends Parasitol. 2018;34:1038–1055. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

