Action Observation Network Activity Related to Object-Directed and Socially-Directed Actions in Adolescents
- PMID: 36347621
- PMCID: PMC9838701
- DOI: 10.1523/JNEUROSCI.1602-20.2022
Action Observation Network Activity Related to Object-Directed and Socially-Directed Actions in Adolescents
Abstract
The human action observation network (AON) encompasses brain areas consistently engaged when we observe other's actions. Although the core nodes of the AON are present from childhood, it is not known to what extent they are sensitive to different action features during development. Because social cognitive abilities continue to mature during adolescence, the AON response to socially-oriented actions, but not to object-related actions, may differ in adolescents and adults. To test this hypothesis, we scanned with functional magnetic resonance imaging (fMRI) male and female typically-developing teenagers (n = 28; 13 females) and adults (n = 25; 14 females) while they passively watched videos of manual actions varying along two dimensions: sociality (i.e., directed toward another person or not) and transitivity (i.e., involving an object or not). We found that action observation recruited the same fronto-parietal and occipito-temporal regions in adults and adolescents. The modulation of voxel-wise activity according to the social or transitive nature of the action was similar in both groups of participants. Multivariate pattern analysis, however, revealed that decoding accuracies in intraparietal sulcus (IPS)/superior parietal lobe (SPL) for both sociality and transitivity were lower for adolescents compared with adults. In addition, in the lateral occipital temporal cortex (LOTC), generalization of decoding across the orthogonal dimension was lower for sociality only in adolescents. These findings indicate that the representation of the content of others' actions, and in particular their social dimension, in the adolescent AON is still not as robust as in adults.SIGNIFICANCE STATEMENT The activity of the action observation network (AON) in the human brain is modulated according to the purpose of the observed action, in particular the extent to which it involves interaction with an object or with another person. How this conceptual representation of actions is implemented during development is largely unknown. Here, using multivoxel pattern analysis (MVPA) of functional magnetic resonance imaging (fMRI) data, we discovered that, while the action observation network is in place in adolescence, the fine-grain organization of its posterior regions is less robust than in adults to decode the abstract social dimensions of an action. This finding highlights the late maturation of social processing in the human brain.
Keywords: action observation; adolescence; fMRI; occipito-temporal cortex; parietal cortex; social actions.
Copyright © 2023 the authors.
Figures





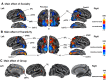

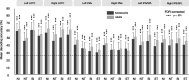
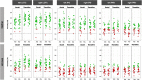
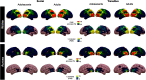
References
-
- Benjamini Y, Yekutieli D (2001) The control of the false discovery rate in multiple testing under dependency. Ann Stat 29:1165–1188.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
