Plasma exosomes generated by ischaemic preconditioning are cardioprotective in a rat heart failure model
- PMID: 36347723
- PMCID: PMC9875906
- DOI: 10.1016/j.bja.2022.08.040
Plasma exosomes generated by ischaemic preconditioning are cardioprotective in a rat heart failure model
Abstract
Background: Exosomes released into the plasma after brief cardiac ischaemia mediate subsequent cardioprotection. Whether donor exosomes can provide cardioprotection to recipients with chronic heart failure, which confers the highest perioperative risk, is unknown. We examined whether ischaemic preconditioning (IPC)-induced plasma exosomes exerted cardioprotection after their transfer from normal donors to post-infarcted failing hearts.
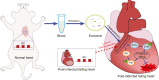
Methods: Plasma exosomes were obtained from adult rats after IPC or sham. An exosome inhibitor GW4869 was administrated before IPC in an in vivo model of ischaemia/reperfusion (I/R) injury in normal rats. The IPC exosomes or control exosomes from normal donor rats were perfused to the normal or post-infarcted failing rat hearts before ischaemia in Langendorff perfusion experiments. Infarct size, cardiac enzymes, cardiac function, and pro-survival kinases were quantified.
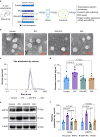
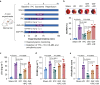
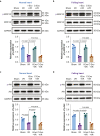
Results: The IPC stimulus increased the release of exosomes, whereas GW4869 inhibited the rise of plasma exosomes. Pre-treatment with GW4869 reversed IPC-mediated cardioprotection against in vivo I/R injury. In the Langendorff perfusion experiments, IPC exosomes from normal donor rats reduced mean infarct size from 41.05 (1.87)% to 31.43 (1.81)% and decreased lactate dehydrogenase activity in the post-infarcted failing rat hearts. IPC exosomes but not control exosomes activated pro-survival kinases in the heart tissues.
Conclusions: Ischaemic preconditioning-induced exosomes from normal rats can restore cardioprotection in heart failure after myocardial infarction, which is associated with activation of pro-survival protein kinases. These results suggest a potential perioperative therapeutic role for ischaemic preconditioning-induced exosomes.
Keywords: cardioprotection; exosome; heart failure; ischaemic preconditioning; pro-survival kinases.
Copyright © 2022 British Journal of Anaesthesia. Published by Elsevier Ltd. All rights reserved.
Figures

Comment in
-
Exosomes as perioperative therapeutics to limit organ injury.Br J Anaesth. 2023 Mar;130(3):248-250. doi: 10.1016/j.bja.2022.12.014. Epub 2023 Jan 20. Br J Anaesth. 2023. PMID: 36682935
References
-
- Hausenloy D.J., Yellon D.M. Ischaemic conditioning and reperfusion injury. Nat Rev Cardiol. 2016;13:193–209. - PubMed
-
- van Waes J.A., Nathoe H.M., de Graaff J.C., et al. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation. 2013;127:2264–2271. - PubMed
-
- Seeger J.P.H., Benda N.M.M., Riksen N.P., et al. Heart failure is associated with exaggerated endothelial ischaemia–reperfusion injury and attenuated effect of ischaemic preconditioning. Eur J Prev Cardiol. 2014;23:33–40. - PubMed
-
- Andersen A., Povlsen J.A., Botker H.E., Nielsen-Kudsk J.E. Right ventricular hypertrophy and failure abolish cardioprotection by ischaemic pre-conditioning. Eur J Heart Fail. 2013;15:1208–1214. - PubMed
-
- Yano T., Miki T., Tanno M., et al. Hypertensive hypertrophied myocardium is vulnerable to infarction and refractory to erythropoietin-induced protection. Hypertension. 2011;57:110–115. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

