The PIP4K2 inhibitor THZ-P1-2 exhibits antileukemia activity by disruption of mitochondrial homeostasis and autophagy
- PMID: 36347832
- PMCID: PMC9643393
- DOI: 10.1038/s41408-022-00747-w
The PIP4K2 inhibitor THZ-P1-2 exhibits antileukemia activity by disruption of mitochondrial homeostasis and autophagy
Abstract
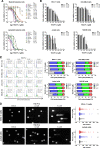
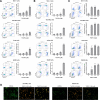
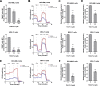
The treatment of acute leukemia is challenging because of the genetic heterogeneity between and within patients. Leukemic stem cells (LSCs) are relatively drug-resistant and frequently relapse. Their plasticity and capacity to adapt to extracellular stress, in which mitochondrial metabolism and autophagy play important roles, further complicates treatment. Genetic models of phosphatidylinositol-5-phosphate 4-kinase type 2 protein (PIP4K2s) inhibition have demonstrated the relevance of these enzymes in mitochondrial homeostasis and autophagic flux. Here, we uncovered the cellular and molecular effects of THZ-P1-2, a pan-inhibitor of PIP4K2s, in acute leukemia cells. THZ-P1-2 reduced cell viability and induced DNA damage, apoptosis, loss of mitochondrial membrane potential, and the accumulation of acidic vesicular organelles. Protein expression analysis revealed that THZ-P1-2 impaired autophagic flux. In addition, THZ-P1-2 induced cell differentiation and showed synergistic effects with venetoclax. In primary leukemia cells, LC-MS/MS-based proteome analysis revealed that sensitivity to THZ-P1-2 is associated with mitochondrial metabolism, cell cycle, cell-of-origin (hematopoietic stem cell and myeloid progenitor), and the TP53 pathway. The minimal effects of THZ-P1-2 observed in healthy CD34+ cells suggest a favorable therapeutic window. Our study provides insights into the pharmacological inhibition of PIP4K2s targeting mitochondrial homeostasis and autophagy, shedding light on a new class of drugs for acute leukemia.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

