The mycotoxin viriditoxin induces leukemia- and lymphoma-specific apoptosis by targeting mitochondrial metabolism
- PMID: 36347842
- PMCID: PMC9643474
- DOI: 10.1038/s41419-022-05356-w
The mycotoxin viriditoxin induces leukemia- and lymphoma-specific apoptosis by targeting mitochondrial metabolism
Abstract
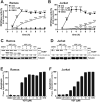
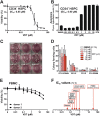
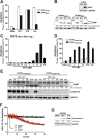
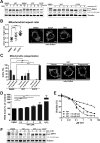
Inhibition of the mitochondrial metabolism offers a promising therapeutic approach for the treatment of cancer. Here, we identify the mycotoxin viriditoxin (VDT), derived from the endophytic fungus Cladosporium cladosporioides, as an interesting candidate for leukemia and lymphoma treatment. VDT displayed a high cytotoxic potential and rapid kinetics of caspase activation in Jurkat leukemia and Ramos lymphoma cells in contrast to solid tumor cells that were affected to a much lesser extent. Most remarkably, human hematopoietic stem and progenitor cells and peripheral blood mononuclear cells derived from healthy donors were profoundly resilient to VDT-induced cytotoxicity. Likewise, the colony-forming capacity was affected only at very high concentrations, which provides a therapeutic window for cancer treatment. Intriguingly, VDT could directly activate the mitochondrial apoptosis pathway in leukemia cells in the presence of antiapoptotic Bcl-2 proteins. The mitochondrial toxicity of VDT was further confirmed by inhibition of mitochondrial respiration, breakdown of the mitochondrial membrane potential (ΔΨm), the release of mitochondrial cytochrome c, generation of reactive oxygen species (ROS), processing of the dynamin-like GTPase OPA1 and subsequent fission of mitochondria. Thus, VDT-mediated targeting of mitochondrial oxidative phosphorylation (OXPHOS) might represent a promising therapeutic approach for the treatment of leukemia and lymphoma without affecting hematopoietic stem and progenitor cells.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Targeting mitochondrial metabolism by the mitotoxin bromoxib in leukemia and lymphoma cells.Cell Commun Signal. 2024 Nov 12;22(1):541. doi: 10.1186/s12964-024-01913-2. Cell Commun Signal. 2024. PMID: 39533399 Free PMC article.
-
Novel meriolin derivatives activate the mitochondrial apoptosis pathway in the presence of antiapoptotic Bcl-2.Cell Death Discov. 2024 Mar 9;10(1):125. doi: 10.1038/s41420-024-01901-y. Cell Death Discov. 2024. PMID: 38461295 Free PMC article.
-
Efficacy of doxorubicin-transferrin conjugate in apoptosis induction in human leukemia cells through reactive oxygen species generation.Cell Oncol (Dordr). 2016 Apr;39(2):107-18. doi: 10.1007/s13402-015-0256-2. Epub 2015 Nov 26. Cell Oncol (Dordr). 2016. PMID: 26611752 Free PMC article.
-
Mitochondria: A Galaxy in the Hematopoietic and Leukemic Stem Cell Universe.Int J Mol Sci. 2020 May 30;21(11):3928. doi: 10.3390/ijms21113928. Int J Mol Sci. 2020. PMID: 32486249 Free PMC article. Review.
-
Oncogenic Mechanisms and Therapeutic Targeting of Metabolism in Leukemia and Lymphoma.Cold Spring Harb Perspect Med. 2021 Jul 1;11(7):a035477. doi: 10.1101/cshperspect.a035477. Cold Spring Harb Perspect Med. 2021. PMID: 32816875 Free PMC article. Review.
Cited by
-
Targeting mitochondrial metabolism by the mitotoxin bromoxib in leukemia and lymphoma cells.Cell Commun Signal. 2024 Nov 12;22(1):541. doi: 10.1186/s12964-024-01913-2. Cell Commun Signal. 2024. PMID: 39533399 Free PMC article.
-
The Golgi stacking protein GRASP55 is targeted by the natural compound prodigiosin.Cell Commun Signal. 2023 Oct 5;21(1):275. doi: 10.1186/s12964-023-01275-1. Cell Commun Signal. 2023. PMID: 37798768 Free PMC article.
-
Anticancer Effect of Mycotoxins From Penicillium aurantiogriseum: Exploration of Natural Product Potential.Int J Microbiol. 2024 Dec 5;2024:5553860. doi: 10.1155/ijm/5553860. eCollection 2024. Int J Microbiol. 2024. PMID: 39669001 Free PMC article. Review.
-
The High-Efficiency Degradation of Multiple Mycotoxins by Lac-W Laccase in the Presence of Mediators.Toxins (Basel). 2024 Nov 4;16(11):477. doi: 10.3390/toxins16110477. Toxins (Basel). 2024. PMID: 39591232 Free PMC article.
-
Design, Synthesis, and Anti-Leukemic Evaluation of a Series of Dianilinopyrimidines by Regulating the Ras/Raf/MEK/ERK and STAT3/c-Myc Pathways.Molecules. 2024 Apr 3;29(7):1597. doi: 10.3390/molecules29071597. Molecules. 2024. PMID: 38611876 Free PMC article.
References
-
- Bosc C, Selak MA, Sarry JE. Resistance is futile: targeting mitochondrial energetics and metabolism to overcome drug resistance in cancer treatment. Cell Metab. 2017;26:705–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

