Evaluation of commercially available fully automated and ELISA-based assays for detecting anti-SARS-CoV-2 neutralizing antibodies
- PMID: 36347859
- PMCID: PMC9643483
- DOI: 10.1038/s41598-022-21317-x
Evaluation of commercially available fully automated and ELISA-based assays for detecting anti-SARS-CoV-2 neutralizing antibodies
Abstract
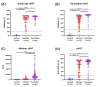
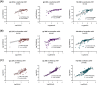
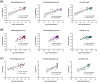
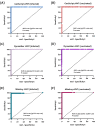
Rapid and accurate measurement of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV2)-specific neutralizing antibodies (nAbs) is paramount for monitoring immunity in infected and vaccinated subjects. The current gold standard relies on pseudovirus neutralization tests which require sophisticated skills and facilities. Alternatively, recent competitive immunoassays measuring anti-SARS-CoV-2 nAbs are proposed as a quick and commercially available surrogate virus neutralization test (sVNT). Here, we report the performance evaluation of three sVNTs, including two ELISA-based assays and an automated bead-based immunoassay for detecting nAbs against SARS-CoV-2. The performance of three sVNTs, including GenScript cPass, Dynamiker, and Mindray NTAb was assessed in samples collected from SARS-CoV-2 infected patients (n = 160), COVID-19 vaccinated individuals (n = 163), and pre-pandemic controls (n = 70). Samples were collected from infected patients and vaccinated individuals 2-24 weeks after symptoms onset or second dose administration. Correlation analysis with pseudovirus neutralization test (pVNT) and immunoassays detecting anti-SARS-CoV-2 binding antibodies was performed. Receiver operating characteristic (ROC) curve analysis was generated to assess the optimal threshold for detecting nAbs by each assay. All three sVNTs showed an excellent performance in terms of specificity (100%) and sensitivity (100%, 97.0%, and 97.1% for GenScript, Dynamiker, and Mindray, respectively) in samples collected from vaccinated subjects. GenScript demonstrated the strongest correlation with pVNT (r = 0.743, R2 = 0.552), followed by Mindray (r = 0.718, R2 = 0.515) and Dynamiker (r = 0.608, R2 = 0.369). Correlation with anti-SARS-CoV-2 binding antibodies was variable, but the strongest correlations were observed between anti-RBD IgG antibodies and Mindray (r = 0.952, R2 = 0.907). ROC curve analyses demonstrated excellent performance for all three sVNT assays in both groups, with an AUC ranging between 0.99 and 1.0 (p < 0.0001). Also, it was shown that the manufacturer's recommended cutoff values could be modified based on the tested cohort without significantly affecting the sVNT performance. The sVNT provides a rapid, low-cost, and scalable alternative to conventional neutralization assays for measuring and expanding nAbs testing across various research and clinical settings. Also, it could aid in evaluating actual protective immunity at the population level and assessing vaccine effectiveness to lay a foundation for boosters' requirements.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Importance, Applications and Features of Assays Measuring SARS-CoV-2 Neutralizing Antibodies.Int J Mol Sci. 2023 Mar 10;24(6):5352. doi: 10.3390/ijms24065352. Int J Mol Sci. 2023. PMID: 36982424 Free PMC article. Review.
-
Can commercial automated immunoassays be utilized to predict neutralizing antibodies after SARS-CoV-2 infection? A comparative study between three different assays.Front Biosci (Landmark Ed). 2021 Jul 30;26(7):198-206. doi: 10.52586/4934. Front Biosci (Landmark Ed). 2021. PMID: 34340267
-
Comprehensive Comparison of Seven SARS-CoV-2-Specific Surrogate Virus Neutralization and Anti-Spike IgG Antibody Assays Using a Live-Virus Neutralization Assay as a Reference.Microbiol Spectr. 2023 Feb 14;11(1):e0231422. doi: 10.1128/spectrum.02314-22. Epub 2023 Jan 9. Microbiol Spectr. 2023. PMID: 36622205 Free PMC article.
-
Evaluation of the correlation between the access SARS-CoV-2 IgM and IgG II antibody tests with the SARS-CoV-2 surrogate virus neutralization test.J Med Virol. 2022 Jan;94(1):335-341. doi: 10.1002/jmv.27338. Epub 2021 Oct 5. J Med Virol. 2022. PMID: 34524695 Free PMC article.
-
Overview of Neutralization Assays and International Standard for Detecting SARS-CoV-2 Neutralizing Antibody.Viruses. 2022 Jul 18;14(7):1560. doi: 10.3390/v14071560. Viruses. 2022. PMID: 35891540 Free PMC article. Review.
Cited by
-
The Detection of SARS-CoV-2 Antibodies in an Exposed Human Population Is Biased by the Immunoassay Used: Implications in Serosurveillance.Pathogens. 2023 Nov 16;12(11):1360. doi: 10.3390/pathogens12111360. Pathogens. 2023. PMID: 38003824 Free PMC article.
-
Assessment of Broadly Reactive Responses in Patients With MERS-CoV Infection and SARS-CoV-2 Vaccination.JAMA Netw Open. 2023 Jun 1;6(6):e2319222. doi: 10.1001/jamanetworkopen.2023.19222. JAMA Netw Open. 2023. PMID: 37389876 Free PMC article.
-
Importance, Applications and Features of Assays Measuring SARS-CoV-2 Neutralizing Antibodies.Int J Mol Sci. 2023 Mar 10;24(6):5352. doi: 10.3390/ijms24065352. Int J Mol Sci. 2023. PMID: 36982424 Free PMC article. Review.
-
Follow-Up and Comparative Assessment of SARS-CoV-2 IgA, IgG, Neutralizing, and Total Antibody Responses After BNT162b2 or mRNA-1273 Heterologous Booster Vaccination.Influenza Other Respir Viruses. 2024 May;18(5):e13290. doi: 10.1111/irv.13290. Influenza Other Respir Viruses. 2024. PMID: 38706402 Free PMC article.
-
Antibody-dependent enhancement of SARS-CoV-2, the impact of variants and vaccination.Hum Vaccin Immunother. 2025 Dec;21(1):2505356. doi: 10.1080/21645515.2025.2505356. Epub 2025 May 24. Hum Vaccin Immunother. 2025. PMID: 40411306 Free PMC article.
References
-
- CDC, U., Interim Guidelines for COVID-19 Antibody Testing. (2022) https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-g....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

