Discovery of a small molecule that selectively destabilizes Cryptochrome 1 and enhances life span in p53 knockout mice
- PMID: 36347873
- PMCID: PMC9643396
- DOI: 10.1038/s41467-022-34582-1
Discovery of a small molecule that selectively destabilizes Cryptochrome 1 and enhances life span in p53 knockout mice
Abstract
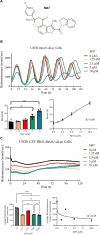
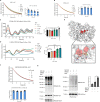
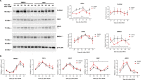
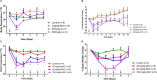
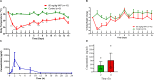
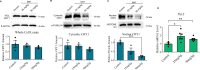
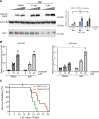
Cryptochromes are negative transcriptional regulators of the circadian clock in mammals. It is not clear how reducing the level of endogenous CRY1 in mammals will affect circadian rhythm and the relation of such a decrease with apoptosis. Here, we discovered a molecule (M47) that destabilizes Cryptochrome 1 (CRY1) both in vitro and in vivo. The M47 selectively enhanced the degradation rate of CRY1 by increasing its ubiquitination and resulted in increasing the circadian period length of U2OS Bmal1-dLuc cells. In addition, subcellular fractionation studies from mice liver indicated that M47 increased degradation of the CRY1 in the nucleus. Furthermore, M47-mediated CRY1 reduction enhanced oxaliplatin-induced apoptosis in Ras-transformed p53 null fibroblast cells. Systemic repetitive administration of M47 increased the median lifespan of p53-/- mice by ~25%. Collectively our data suggest that M47 is a promising molecule to treat forms of cancer depending on the p53 mutation.
© 2022. The Author(s).
Conflict of interest statement
The M47 studied in this paper is awarded of the following patent: WO2021137771A1 (World Intellectual Property Organization, Patent Cooperation Treaty). S.G., M.T., and I.H.K. are inventors in these patent applications, while Koc University is the assignee. The title of the patent is “11-(4-chlorophenyl)−4-(2,3-dihydro-1h-indole-1-carbonyl)−3,11-dimethyl-5,10,dioxatricyclo[7.4.0.0,2,6,]trideca-1,3,6,8-tetraen-13-one and derivatives as destabilizer of cry1 for the treatment of circadian rhythm associated diseases and disorders”. The invention provided an anticancer molecule against cancers harboring p53 mutations. The other authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

